- Departments of Emergency Medicine, University of Maryland Shore Medical Center, Easton, United States.
- Departments of Neurosurgery, University of Maryland Shore Medical Center, Easton, United States.
- Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, United States.
Correspondence Address:
Khalid Kurtom
Departments of Neurosurgery, University of Maryland Shore Medical Center, Easton, United States.
Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, United States.
DOI:10.25259/SNI-25-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eric Klotz, Wendy Towers, Khalid Kurtom. Minimizing cortical disturbance to access ventricular subependymoma – A novel approach utilizing spinal minimally invasive tubular retractor system. 07-Jun-2019;10:95
How to cite this URL: Eric Klotz, Wendy Towers, Khalid Kurtom. Minimizing cortical disturbance to access ventricular subependymoma – A novel approach utilizing spinal minimally invasive tubular retractor system. 07-Jun-2019;10:95. Available from: http://surgicalneurologyint.com/surgicalint-articles/9351/
Abstract
Background:Subependymomas are rare benign tumors found primarily in the lateral and fourth ventricles. Patients become symptomatic when the tumor obstructs cerebrospinal fluid pathways. We present a novel minimally invasive technique for lateral ventricular subependymoma resection.
Case Description:A 57-year-old male presented after a period of progressive ataxia, right upper extremity tremor, and syncopal events. Emergent non-contrast computed tomography of the brain demonstrated a lobulated mass in the left lateral ventricle causing moderate-to-severe obstructive hydrocephalus. Emergent ventriculostomy was placed as a temporizing measure. Subsequent magnetic resonance imaging (MRI) illustrated a large benign appearing mass causing obstruction of the left foramen of Monroe. A small craniotomy was performed utilizing previous ventriculostomy twist hole. The left lateral ventricle was accessed through sequential dilation of ventriculostomy tract using a minimally invasive spine surgery tubular system. Tumor was resected en bloc under microscopic assistance. The patient had an excellent outcome with return to baseline mental status and was discharged from the hospital postoperative day 1. Follow-up MRI demonstrated gross total resection of the mass and decreasing lateral ventricle hydrocephalus with minimal cortical disturbance.
Conclusion:A minimally invasive tubular system approach to ventricular tumors can be utilized to minimize cortical resection and brain retraction. Minimally invasive surgery also has the potential to decrease the length of stay and enhance postoperative recovery.
Keywords: Intraventricular tumors, Minimally invasive spine surgery, Minimally invasive spine tubular retractor, Subependymoma
BACKGROUND AND IMPORTANCE
Subependymomas are benign intraventricular slow-growing tumors found mostly in the lateral and fourth ventricles.[
CLINICAL PRESENTATION/CASE REPORT
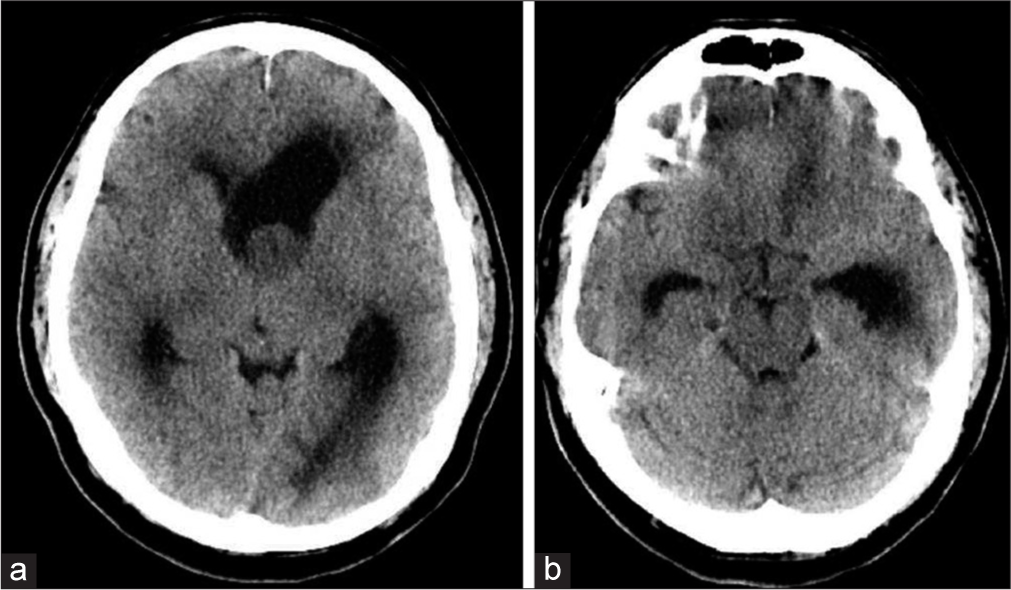
A 57-year-old male presented to the emergency department after 2 weeks of the right upper extremity tremor, progressive ataxia, and a syncopal event. Neurologic examination was significant only for confusion and a resting tremor of his right upper extremity. Non-contrast brain computed tomography (CT) demonstrated a left lateral ventricle lobulated soft tissue density mass measuring 2.0 cm × 2.2 cm causing moderate-to-severe obstructive hydrocephalus at the foramen of Monroe [
Figure 2
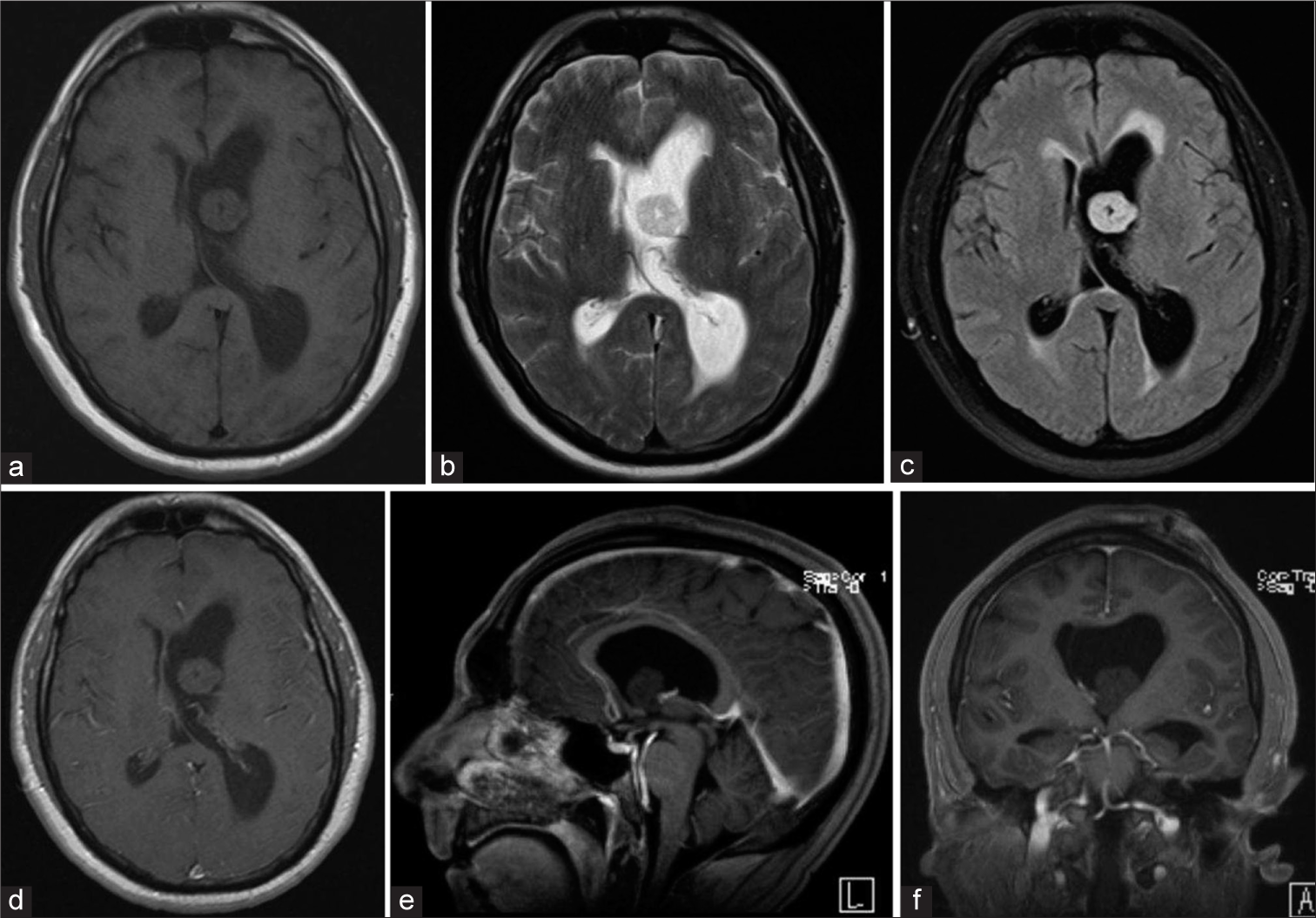
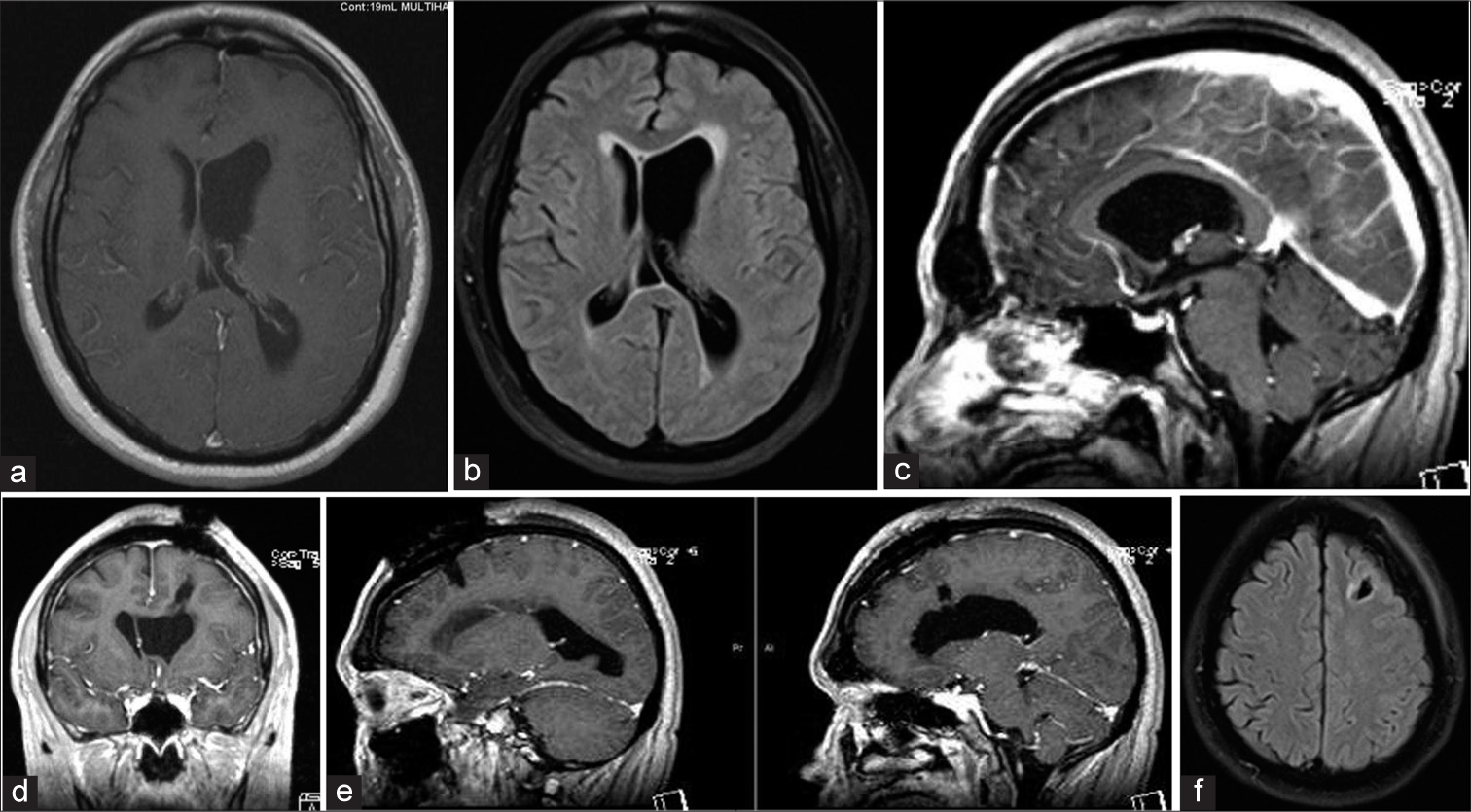
Magnetic resonance imaging brain with gadolinium demonstrating a large benign appearing mass causing obstruction of the left foramen of Monroe, (a) TI hypointense mass, (b) T2 hypointense mass, (c) Flair hyperintense mass with transependymal edema, (d) Axial T1 w/gad hypointense mass without evidence of enhancement, (e) Sagittal T1 w/gad non-enhancing mass obstructing foramen of Monroe, (f) Coronal T1 w/gad non-enhancing mass obstructing lateral ventricle with left ventricle hypertrophy and rightward septal shift.
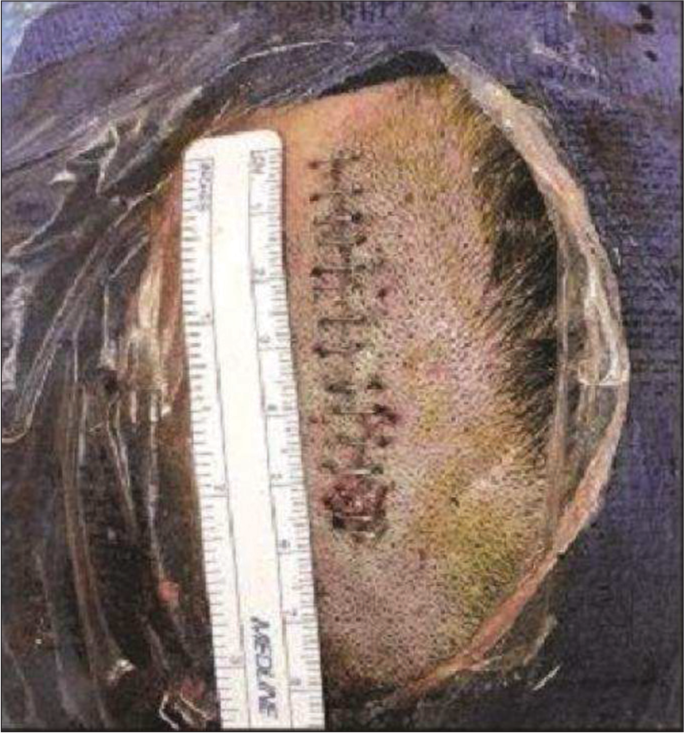
The patient was placed under general anesthesia in a supine position with the head slightly flexed. A two-inch straight incision was made over the left frontal region incorporating the ventriculostomy puncture site [
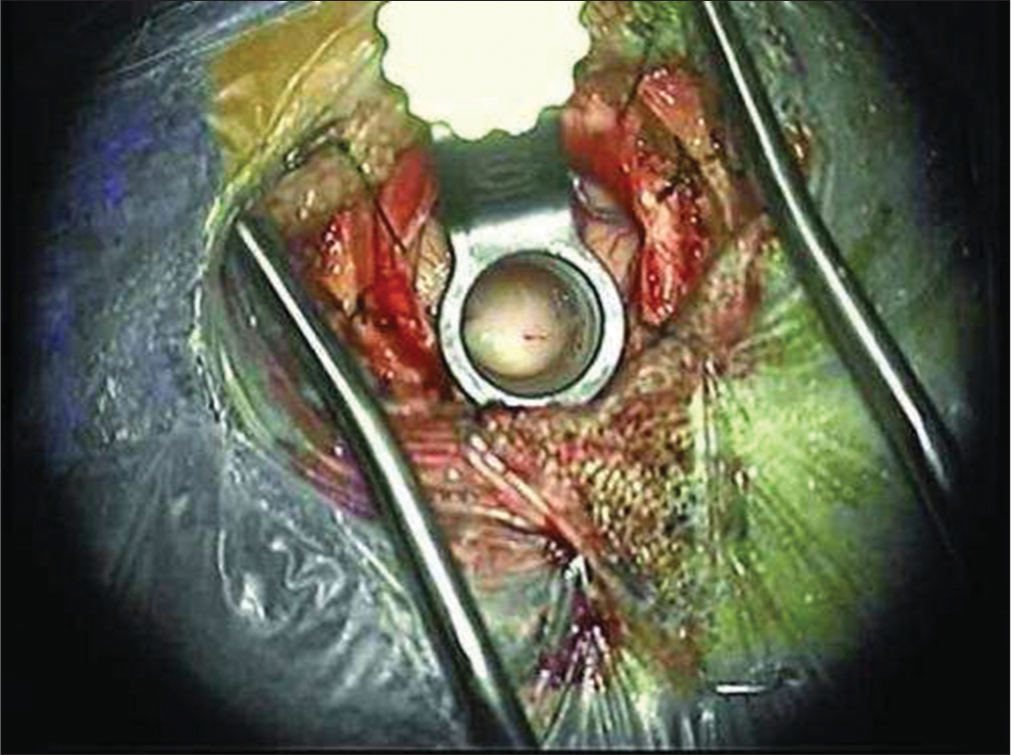
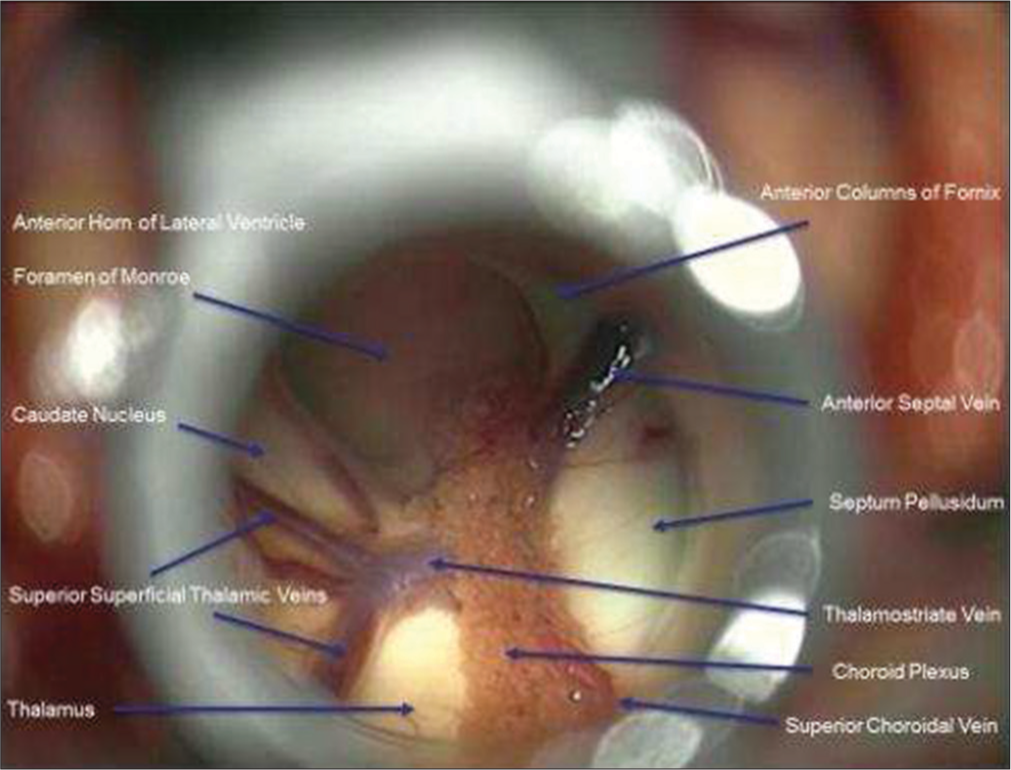
A small incision was made into the mass to obtain biopsy. Internal debulking was allowed for manipulation of the mass. A cottonoid covered the Foramen of Monroe to isolate the lateral ventricle in case of intraoperative bleeding. Bipolar electrocautery and micro scissors were used to transect the pedicle from the lateral ventricular wall. The mass was then removed en bloc. The ventricular anatomy was examined to confirm open CSF pathway before removal of the tubular retractor [
The patient had immediate return to baseline mental status and was discharged from the hospital postoperative day 1. Follow-up MRI demonstrated gross total resection of the mass and decreased lateral ventricle hydrocephalus with minimal cortical disturbance [
Figure 6
Postoperative magnetic resonance imaging brain w/gad demonstrating total resection of the mass and decreasing lateral ventricle hydrocephalus with minimal cortical disturbance, (a) Axial T1 w/gad with decreased left lateral ventricular hydrocephalus and gross total resection of ventricular mass, (b) Flair demonstrating reduced transependymal edema, (c) Sagittal T1 w/gad with gadolinium with patent foramen of Monroe after complete resection of intraventricular mass, (d) Coronal T1 w/gad illustrating the trajectory of tubular channel through cortex, (e) Sagittal T1 w/gad view of tubular channel through the cortex, (f) Axial flair view minimal cortical disruption.
DISCUSSION
Subependymomas are extremely rare benign and slow-growing tumors usually found in the ventricular system. Incidence has traditionally been reported as 0.5–0.7% of intracranial tumors; however, recent studies have found a much lower frequency of 0.07% of cases.[
On CT, the mass will typically appear hypodense or isodense to the surrounding tissue and without calcification.[
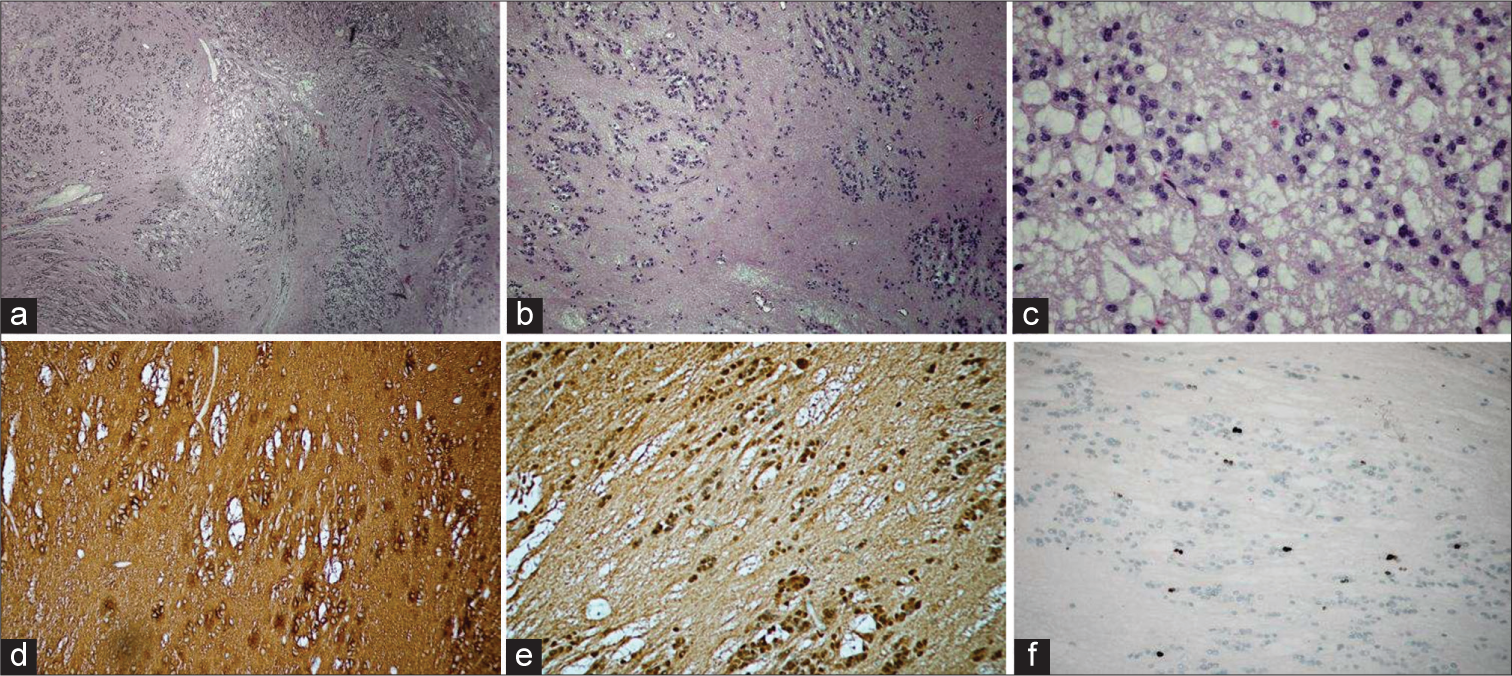
Pathologically, these tumors are most often pure subependymomas; however, they can be mixed subependymoma-ependymoma tumors as well as subependymoma-astrocytoma tumors.[
Subependymomas are a surgical disease with total resection being considered curative. There are varying opinions on the role of radiotherapy; however, there is no significant association with increasing survival to support its routine use. Authors conclude that radiotherapy may be considered if a subependymoma is a mixed tumor type, if a subtotal resection is performed, or if a patient remains symptomatic after a subtotal resection.[
When considering access to the tumor, traditionally a transcortical or transcallosal approach is undertaken. The former tends to have a higher rate of postoperative seizure and subdural fluid collections. Transcallosal approaches run the risk of hemiparesis, postoperative mutism and classical disconnection syndrome. Surgical outcome depends predominantly on the patient’s preoperative deficits rather than the approach taken.[
CONCLUSION
Subependymomas are indolent benign tumors found primarily in the lateral and fourth ventricles and become symptomatic when CSF flow is obstructed. A minimally invasive spine tubular system approach to ventricular tumors can be utilized to minimize cortical resection and brain retraction. Minimally invasive surgery also has the potential to decrease the length of stay and enhance postoperative recovery.
Patient consent
The patient has consented to the submission of the case report for submission to the journal.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
1. Artico M, Bardella L, Ciappetta P, Raco A. Surgical treatment of subependymomas of the central nervous system. Report of 8 cases and review of the literature. Acta Neurochir (Wien). 1989. 98: 25-31
2. Asgari S, Engelhorn T, Brondics A, Sandalcioglu IE, Stolke D. Transcortical or transcallosal approach to ventricle-associated lesions: A clinical study on the prognostic role of surgical approach. Neurosurg Rev. 2003. 26: 192-7
3. Bi Z, Ren X, Zhang J, Jia W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg. 2015. 122: 49-60
4. Chiechi MV, Smirniotopoulos JG, Jones RV. Intracranial subependymomas: CT and MR imaging features in 24 cases. AJR Am J Roentgenol. 1995. 165: 1245-50
5. D’angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lateral ventricle tumors: Surgical strategies according to tumor origin and development-a series of 72 cases. Neurosurg. 2005. 56: 36-45
6. Eliyas JK, Glynn R, Kulwin CG, Rovin R, Young R, Alzate J. Minimally invasive transsulcal resection of intraventricular and periventricular lesions through a tubular retractor system: Multicentric experience and results. World Neurosurg. 2016. 90: 556-64
7. Hernández-Durán S, Yeh-Hsieh TY, Salazar-Araya C. Pedunculated intraventricular subependymoma: Review of the literature and illustration of classical presentation through a clinical case. Surg Neurol Int. 2014. 5: 117-
8. Jain A, Amin AG, Jain P, Burger P, Jallo GI, Lim M. Subependymoma: Clinical features and surgical outcomes. Neurol Res. 2012. 34: 677-84
9. Maiuri F, Gangemi M, Iaconetta G, Signorelli F, Del Basso De Caro M. Symptomatic subependymomas of the lateral ventricles. Report of eight cases. Clin Neurol Neurosurg. 1997. 99: 17-22
10. Matsumura A, Ahyai A, Hori A. Symptomatic subependymoma with nuclear polymorphism. Neurosurg Rev. 1987. 10: 291-3
11. Matsumura A, Ahyai A, Hori A, Schaake T. Intracerebral subependymomas. Clinical and neuropathological analyses with special reference to the possible existence of a less benign variant. Acta Neurochir (Wien). 1989. 96: 15-25
12. Ragel BT, Osborn AG, Whang K, Townsend JJ, Jensen RL, Couldwell WT. Subependymomas: An analysis of clinical and imaging features. Neurosurgery. 2006. 58: 881-90
13. Rath TJ, Sundgren PC, Brahma B, Lieberman AP, Chandler WF, Gebarski SS. Massive symptomatic subependymoma of the lateral ventricles: Case report and review of the literature. Neuroradiology. 2005. 47: 183-8
14. Scheinker M. Subependymoma: A newly recognized tumor of subependymal derivation. J Neurosurg. 1945. 2: 232-240
15. Scheithauer BW. Symptomatic subependymoma. Report of 21 cases with review of the literature. J Neurosurg. 1978. 49: 689-96
16. Varma A, Giraldi D, Mills S, Brodbelt AR, Jenkinson MD. Surgical management and long-term outcome of intracranial subependymoma. Acta Neurochir (Wien). 2018. 160: 1793-9