-
PDF
- Split View
-
Views
-
Cite
Cite
Paul V. R. Snelgrove, Getting to the Bottom of Marine Biodiversity: Sedimentary Habitats: Ocean bottoms are the most widespread habitat on Earth and support high biodiversity and key ecosystem services, BioScience, Volume 49, Issue 2, February 1999, Pages 129–138, https://doi.org/10.2307/1313538
Close - Share Icon Share
The oceans encompass habitats ranging from highly productive coastal regions to lightless, high-pressure, and low-temperature deep-sea environments. The benthic (bottom-living) species that reside within the sediments in these habitats form one of the richest species pools in the oceans and perhaps on Earth. Even though 70.8% of the earth is covered by oceans, and most ocean floor is covered by sediments, there is still much to learn about biodiversity in marine sediments. The major reasons for the gaps in knowledge are logistics and effort. Approximately 65.5% of the planet is covered by ocean that is greater than 130 m in depth (i.e., the approximate depth limit of the continental shelf) and is accessible only by sub-mersibles or remote-sampling gear. Even the remaining shallow areas (i.e., approximately 5% of the earth's surface) present challenges in terms of ship availability and cost, as well as loss of experiments and ship time to weather.
Despite these logistical difficulties, it is important to improve our understanding of biodiversity in marine sediments. In this article, I describe the biodiversity of organisms residing in the marine sedimentary environment, the patterns that have been observed, why these patterns are thought to exist, and why we should care. Further discussions of marine biodiversity (NRC 1995), and biodiversity in marine sediments in particular (Snelgrove et al. 1997), may be found elsewhere.
The oceans harbor tremendous biological diversity. Of the 29 nonsymbiont animal phyla that have been described so far, all but one has living representatives in the ocean, and 13 are represented only in the oceans; all of these phyla have representatives in the benthos, and most have representatives in marine sediments. Most of the species diversity in marine ecosystems consists of invertebrates residing in (infauna) and on (epifauna) sediments. These invertebrates include large animals (megafauna), such as scallops and crabs, that can be identified from bottom photographs. However, most species are polychaetes, crustaceans, mollusks (macrofauna, larger than 300 μμm), and tiny crustaceans and nematodes (meiofauna, 44–300 μμm). In addition, there are the poorly known microbiota (smaller than 44 μμm), which include bacteria and protists.
Living in marine sediments
Organisms that live in marine sediments face numerous challenges. Except in the shallowest areas, where there is sufficient light to allow photosynthesis at the bottom, most sedimentary organisms are dependent on phytoplankton and other organic material sinking down from surface waters above. The spatial decoupling of production from most marine benthic environments makes these environments fundamentally different from those of terrestrial (Wall and Moore 1999) and freshwater (Covich et al. 1999) benthos. With increasing water depth, the amount of material reaching the bottom decreases; most deep-sea sedimentary environments are thought to be food limited.
To take advantage of whatever food is present, some organisms (suspension feeders) are able to remove suspended particles from near-bottom water; others (deposit feeders) rely on particles that have settled onto the bottom. Some mega- and macrofaunal species suspension feed, many deposit feed, and a few macrofaunal species do both. Meiofauna and microbiota depend on deposited organic material. The mobility of many benthic organisms is relatively limited; many are sessile, and others have only limited mobility within sediments. As a result, many benthic species rely completely on the water above them to supply food.
Water also supplies oxygen, a basic requirement for most organisms residing in sediments. As organisms respire and use up oxygen, sediments can quickly become anoxic (particularly where large amounts of organic matter sink from surface waters) and therefore inhospitable for the majority of species (e.g., Rhoads et al. 1978). Macrofauna move through, or move, the sediment as they feed; this bioturbation enhances movement of water between sediment grains and increases sediment oxy-genation (Rhoads 1974). However, the sediment is often anoxic below the top few millimeters to centimeters, and the vast majority of organisms that require oxygen must therefore either live close to the sediment surface or maintain a burrow of some sort to allow water circulation and oxygenation. But living near the surface of sediments creates problems. Organisms that live near the surface are more vulnerable to predators that feed on sedimentary organisms (e.g., VanBlaricom 1982, Carlson et al. 1997), and sediments can be mobilized in strong currents, causing organisms to be be swept along or buried (e.g., Thistle et al. 1985). Overall, it would appear that marine sediments, particularly those in the deep sea, are an unfavorable environment in which to live—yet the rich diversity of species that reside there suggests otherwise.
Biodiversity in marine sediments
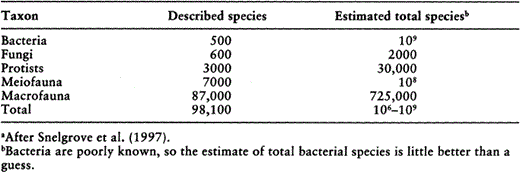
Few marine sedimentary habitats have been well sampled, and previously undescribed species are often found when new studies are undertaken. For example, 64% of the poly-chaete taxa identified in a recent deep-sea study were new to science (Grassle and Maciolek 1992). And even Georges Bank, a commercially important and relatively well studied area off of the eastern United States, yielded 33% new polychaete species in a recent study (James Blake personal communication, as cited in NRC 1995). Table 1 summarizes a recent synthesis of estimated numbers of described and projected numbers of species in marine sediments (Snelgrove et al. 1997). The projected numbers are essentially educated guesses, but even if they are within an order of magnitude of the actual values, they suggest that fewer than 1% of marine species are presently known. Thus, as in terrestrial (Behan-Pelletier and Newton 1999, Wall and Moore 1999) and aquatic (Covich et al. 1999) systems, there are significant numbers of undescribed species in marine sediments, but the problem of describing species in marine sediments may be exacerbated by the inaccessibility of these habitats.
Numbers of described and projected species in marine sedimentary habitats.a
Moreover, biologists are even beginning to discover problems with identification of species that they thought they knew. For example, the blue mussel, Mytilus edulis, which was previously thought to occur broadly across the North Atlantic, is now known to be three overlapping species (McDonald et al. 1991). At least two of these species differ in physiology and contaminant uptake (Lobel et al. 1990). Given that this “species” has been the focus of an international study on pollutant uptake, this shortcoming in species delineation is serious. The pollution indicator Capitella “capitata” has also been shown to be a species complex, whose members have markedly different reproductive strategies (Grassle and Grassle 1976). Similar taxonomic problems are emerging for many other marine species (see Knowlton 1993), and any hope for broad understanding and predictive capability in marine systems rests on the resolution of such problems.
The understanding of patterns of marine diversity changed substantially in the late 1960s with a landmark study (Sanders 1968) that compared diversity in different marine sedimentary habitats. Not surprisingly, Sanders found that physiologically stressful environments, such as estuaries, have low species diversity; shallow-water sedimentary habitats outside the tropics have somewhat higher diversity; and tropical sediments are species rich. What came as a great surprise, however, was the finding that deep-sea sediments are very diverse; for a habitat previously considered species poor, this finding was quite a revelation. More recently, Grassle and Maciolek (1992) used data from an intensive deep-sea sampling program off the eastern United States to estimate that deep-sea sediments could contain as many as 10 million species, a number that rivals estimates for tropical rain forests. This estimate generated tremendous debate about the assumptions that must be made to scale up species estimates from a relatively small area to ocean basins; in fact, May (1992) used the ratio of undescribed to known species in Grassle and Maciolek's (1992) study to estimate that there are fewer than 500,000 species in deep-sea sediments. Conversely, Poore and Wilson (1993) suggested that Grassle and Maciolek's (1992) study area was lower in diversity than some areas of the Pacific and therefore may lead to underestimates of total species numbers. And Lambshead (1993) suggested that the diversity of meiofauna may exceed that of macrofauna, so that if Grassle and Maciolek's estimate is correct, there may be on the order of 100 million nematode species alone in the deep sea. Regardless of which estimate is correct, it is clear that there are many species in marine sediments, most of which are undescribed.
There has also been considerable debate regarding global patterns of diversity in marine sediments. Several studies have documented a latitudinal gradient in shallow-water benthos (see Roy et al. 1998 and references therein), with decreasing diversity toward the poles. The presumed mechanisms of higher diversity in the tropics relate to the effect of solar input on temperature, productivity, and rates of evolution (Jablonski 1993, Roy et al. 1998). Similarly, it has been suggested that tropical continental slope environments are more diverse than their temperate and polar counterparts because changing productivity toward the poles depresses diversity (Poore and Wilson 1993, Rex et al. 1993). Interestingly, data on nema-todes suggests that no latitudinal trend exists (Boucher and Lambshead 1995), raising the intriguing possibility that different groups of marine organisms may be affected by fundamentally different processes. A commonly observed parabolic relationship of diversity with depth (e.g., Rex 1981), in which diversity is highest on the mid- to lower continental slope, may not be universal. Several studies have suggested that the deep ocean abyssal plain may in fact harbor comparable or greater diversity than slope environments (Poore and Wilson 1993, Boucher and Lambshead 1995).
Another generalization that has been questioned (Gray et al. 1997) is that diversity is higher in deep-sea than in shallow-water areas (Sanders 1968, Grassle and Maciolek 1992). Data from the Australian shelf in particular (Gray et al. 1997) indicate that diverse shallow-water environments are not confined to tropical latitudes. The variability in diversity patterns most likely reflects differences in the study areas, the available data sets and assumptions used in analyses, and the specific taxonomic groups examined. Perhaps most important, it reflects the need to improve sampling coverage and to move beyond the historical North Atlantic sampling and perception bias. When data from other areas of the ocean and for multiple taxa become available through increased sampling efforts, it may be possible to improve on depth- and latitude-related generalities and their causes.
A number of other trends have been noted in shallow-water systems. Diversity is lowest in physically extreme environments, such as estuaries (Sanders 1968), eutrophied areas (Pearson and Rosenberg 1978), and high-energy regions with low organic content (Whitlatch 1977). Areas with greater diversity in sediment grain size may have higher species diversity (Whitlatch 1977), and diversity of macrofauna in seagrass bed sediments is higher than in adjacent open areas (see Peterson 1979).
Given that patterns in diversity exist in marine systems, an obvious question is: What processes are important in establishing and maintaining these patterns? This issue is best considered separately for shallow-water (coastal and continental shelf habitats) and deep-sea (habitats beyond continental shelves, or approximately 130 m in depth) ecosystems. This division is appropriate because different processes may be important in the different environments, although the discontinuity in approach and knowledge may also simply reflect the biases of the scientists involved. Shallow-water studies have traditionally focused on describing and understanding patterns of species distributions, and what is known of biodiversity in shallow-water systems has been learned indirectly. In deep-sea systems, in which patterns are less obvious, studies have tended to focus on biodiversity per se. Upper-slope environments are even less well sampled than some deeper areas, so there is a geographic as well as a conceptual discontinuity.
Maintenance of shallow-water diversity
Distribution patterns of individual species of shallow-water sedimentary fauna are determined largely by temperature, salinity, depth, surface productivity, and sediment dynamics over broad scales and by biological interactions, sediment geochemistry, and near-bed flow processes at finer scales. Particularly over broad scales, geologic history plays a major role in patterns of distribution (Jablonski and Sepkowski 1996), although I will focus on ecological rather than evolutionary scales. The dependence on temperature, salinity, and depth are easily understood in terms of physiological constraints; many species have specific tolerances to temperature, salinity, and pressure that have to do with osmotic balance and enzyme function. These physiological constraints contribute to the reduced diversity of estuaries and other highly variable, and thus physically challenging, environments. Given that many organisms derive their nutrition from sediment-associated food particles, higher diversity in sediments of diverse grain size might also be predicted (Whitlatch 1977).
Many species have a complex relationship with the sedimentary environment. Generally speaking, suspension feeders tend to be most abundant in high-energy environments, and deposit feeders are most abundant in depositional areas with fine-grained, muddy sediments. But contrasting these environments in terms of how they determine infau-nal pattern is complex because many important variables vary with flow regime (Snelgrove and Butman 1994). High-energy environments are typically sandy, with strong bottom flows and horizontal flux of food and perhaps settling larvae. Sediment grain size is large, and organic content and microbial content tend to be low. High energy produced by waves and strong currents moves sediments and some organisms. Low-energy environments are often muddy, with weak flows and low horizontal but greater vertical flux of food, fine sediments, and (potentially) larvae.
By mechanisms that are not yet fully understood, these flow-, nutrition-, and substrate-related variables contribute to patterns in species distributions that are fairly consistent in time and space. The challenge is to determine which mechanisms are most important in creating and maintaining pattern. Understanding how patterns in individual species are maintained is a key prerequisite to understanding biodiversity patterns, and some of the advances made in this area are reviewed below.
Most of what is known about shallow-water diversity has been learned from experiments designed to determine the impacts of individual species on other species or from observational data. Among the most relevant of these experiments for understanding regulation of diversity are those that test the impacts of predators on individual species and those that examine the importance of competition in soft-sediment systems. Indeed, experimental approaches in soft-sediment systems have been heavily influenced by studies of rocky intertidal areas, which have demonstrated the critical importance of keystone predators in maintaining diversity and community structure (Paine 1966). Data from the majority of studies in soft-sediment systems (reviewed by Peterson 1979) suggest that interspecific competition is probably not a major structuring force in sedimentary communities but that predation can be important. In reviewing predator exclusion experiments, Peterson (1979) found that species richness in sediments tended to increase when predators were excluded. He also found that species richness in seagrass beds exceeded that in ambient sediments, perhaps because of the predator refuge that seagrasses provide.
Numerous studies of changes resulting from foraging predators suggest that foragers have major impacts on densities of dominant taxa but little effect on the relative abundances of species (e.g., VanBlaricom 1982). But predation effects are not limited to foraging species and their impacts on adult infauna. Indeed, interactions between infaunal adults and settling larvae or recently settled juveniles may be a major structuring force in sedimentary communities. In reviewing the many studies that have been conducted on adult-juvenile interactions, Olafsson et al. (1994) concluded that recruitment success is often inhibited by resident species of both macrofauna and meiofauna; however, the global significance of this effect is difficult to assess from existing data. Although suspension feeders can and do filter settling larvae from near-bottom waters, early postsettlement processes may be more important to recruitment success, given the frequency with which deposit feeders have an impact on suspension feeders.
Biological disturbances, such as bioturbation, may also enhance diversity (Kukert and Smith 1992), although the mechanism is unclear. Bioturbation is also the basis of the trophic group amensalism hypothesis, in which deposit feeders are suggested to constrain distribution patterns in suspension feeders by resuspending sediment that settles and smothers their larvae and clogs their filtering structures (Rhoads and Young 1970). Although this hypothesis is not accepted as a general hypothesis for benthic pattern (Snelgrove and Butman 1994), the interactions that it describes undoubtedly occur in some instances. Biological structures, such as seagrass blades (e.g., Peterson 1979) andpoly-chaete tubes, also have a marked effect on species distribution and abundance; however, linking these effects to biodiversity patterns remains a challenge.
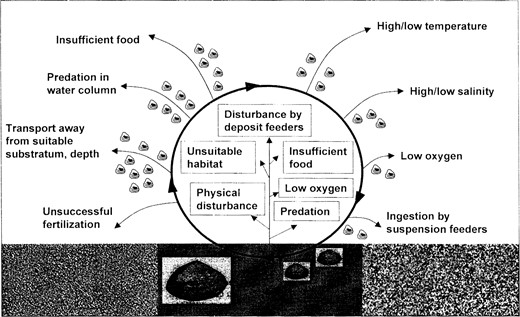
Another factor that may play a major role in establishing pattern is larval supply (Figure 1). Many benthic invertebrates produce plank-tonic larvae that, depending on the taxon, spend hours to months in the plankton before taking up a benthic existence. A major question in marine ecology is how these planktonic larvae, which are often poor swimmers, are able to settle in a suitable habitat. Small-scale laboratory experiments that began in the 1920s suggested that larvae have some capacity to choose among sediment types, perhaps based on organic content. But the scales over which habitat selection behavior may be important are limited, given the relatively weak swimming ability of many larvae. Consequently, these small-scale stillwater experiments may have limited application to nature (Butman 1987).
The challenge of maintaining pattern. Schematic representation of the processes that affect larval settlement of sedimentary invertebrates. The adult bivalve is shown as a large individual living in mud (indicated by solid grey box) but unable to live in other sediments (indicated by grainy boxes). To maintain populations in an area, the bivalve must complete the cycle indicated by the heavy black circle. Lighter lines indicate sources of mortality. Eggs and sperm are spawned into the water column, where some successful fertilizations will occur but many eggs and sperm will be lost. The successfully fertilized eggs become small swimming larvae that suffer heavy losses as a result of transport away from suitable habitat, predation, starvation from lack of appropriate food, and exposure to temperatures or salinities beyond their physiological tolerance. As developing larvae settle toward the bottom, they may encounter hypoxic sediments or predation from suspension-feeding bivalves. Even after the larvae settle and metamorphose into small juveniles, heavy loss is incurred (shown in boxes) because of predators, physical disturbance, low oxygen, insufficient food, unsuitability of sediment, and disturbance by deposit feeders moving through the sediment. Once they have grown past the juvenile stage, mortality is greatly reduced.
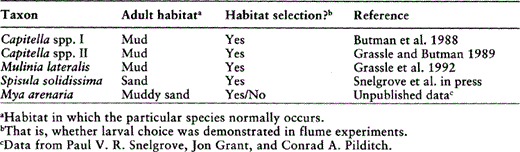
One approach to resolving the importance of habitat selection is to study larval settlement in a laboratory flume. A flume is a recirculating seawater channel that is designed to mimic natural bottom flow but that allows confounding variables such as predators and food supply to be controlled. Over the past few years, several studies of larval settlement have found that species with well-defined distributions with respect to sediment type are also capable of choosing one type of sediment over another as they settle, even in moving water (Table 2). The specific sediment cue to which settling larvae respond is unclear, but organic content is a good candidate for at least some species (Butman et al. 1988). In any case, these results suggest that habitat selection probably plays an important role in shallow-water pattern, although passive transport also regulates delivery of larvae to specific areas (see Butman 1987). How larval ecology relates to maintenance of assemblages and biodiversity remains to be seen.
Summary of laboratory flume experiments to determine whether settling larvae of different species are capable of habitat selection.
In summary, studies from shallow-water environments offer insights into how distributions of individual species are established and maintained, but they have less to say about biodiversity patterns. Existing data suggest that rocky intertidal paradigms may not be applicable to soft-sediment systems and that additional experimental work will be needed to evaluate critically the factors that regulate biodiversity.
Maintenance of deep-sea diversity
Biodiversity in deep-sea ecosystems has generated much interest (e.g., May 1992), in part because of evidence suggesting that they are species rich (Grassle and Maciolek 1992). Why the deep sea is so diverse is a subject of some debate. For some areas of the deep sea, overriding environmental variables, such as low oxygen (Levin and Gage 1998), hydrothermal fluid emission (Dinet et al. 1988), and unusually high productivity (Schaff et al. 1992), depress diversity. The suggestion that the long-term stability of most deep-sea environments has allowed evolution of many specialized species (Sanders 1968) has been questioned based on the lack of evidence for niche specialization and the parabolic diversity-depth relationship that has been observed in some areas (Rex 1981). The potential impact of predators cropping populations below levels at which competitive exclusion would take place has been questioned based on population attributes of deep-sea species (Grassle and Sanders 1973). Indeed, if predation effects in the deep sea are similar to those in shallow water, then reduced predation pressure in the deep sea might actually increase diversity. It has also been hypothesized that small-scale patches of food and disturbance create microhabitats on which different species may specialize and thus avoid competition in a highly food limited environment (Grassle and Sanders 1973). Indeed, carbon flux to the deep sea is now known to be patchy in many areas (e.g., Lampitt 1985), in contrast to the prior concept of homogeneity (Sanders 1968).
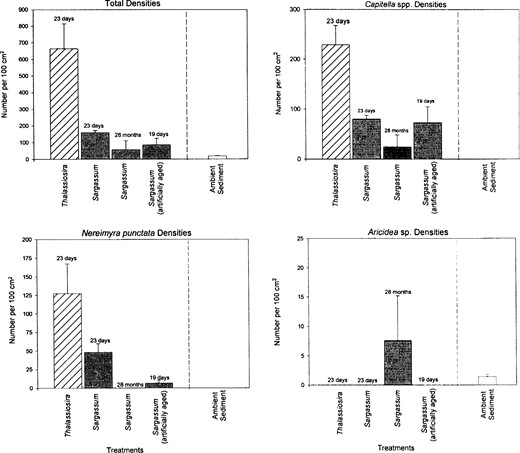
To test the potential role of food patches in the deep sea, sediments enriched with different types of organic matter were deployed for varying periods of time at 900 m in depth near St. Croix in the US Virgin Islands. Different types of organic matter were found to elicit different colonization responses for different species (Figure 2), depending on the type of organic matter (Snelgrove et al. 1992), its state of decomposition, and the duration of deployment (Snelgrove et al. 1996). Thus, small-scale patchiness may enhance deep-sea diversity. However, it is important to note that the numbers of species that respond to these types of disturbance are relatively few, and existing data support this mechanism for only a small subset of deep-sea species. It is possible that appropriate patch types have not been identified for other species, but it is also likely that factors such as productivity and evolutionary history come into play in determining biodiversity patterns.
Deep-sea colonization experiments carried out at 900 m depth south of St. Croix, US Virgin Islands. Three polychaete species (Capitella spp., Nereimyra punctata, and Arichidea sp.) were among the most abundant colonizers. The graphs show the responses of total macrofauna (upper left) and the three polychaete species to different patch types. Two different time periods were compared to test how the species' response to different patch types would change. Ambient sediment refers to densities of macrofauna in natural sediment collected with an ALVIN-style box corer, a metal corer that collects a quantitative sediment sample 10 cm deep and measuring 15××15 cm. All other treatments are densities of animals colonizing 10 cm deep sediment trays with surface areas of 100 cm2. Trays were unenriched or enriched with Sargassum spp. or Thalassiosira algae. Artificially aged Sargassum is algae that was aged before deployment to mimic degradation over a longer time period. Bars denote means, and lines denote 1 SE. Data from Snelgrove et al. (1992, 1996).
Conflicting patterns from different data sets must be resolved to establish any comprehensive paradigm explaining the rich diversity of the deep sea. What is needed is more complete sampling on a global scale, studies that include a broader range of taxa (e.g., macrofauna and meiofauna) within the same study area, and experimental efforts that reveal the most relevant time and spatial sampling scales to document response to different patch types.
Why worry about marine sedimentary diversity?
Even though marine sedimentary ecosystems are not well understood, there are good reasons to assume that their loss could affect the planet and human populations directly. For example, marine organisms provide a tremendous reservoir of natural products that could prove invaluable and irreplaceable by synthetic equivalents. Perhaps more important, a recent study suggested that the oceans provide approximately two-thirds of the $33 trillion worth of ecosystem services that the earth provides (Costanza et al. 1997). Ultimately, however, the arguments for preserving marine sedimentary biodiversity that will carry the greatest weight are those of most immediate concern to human populations. In other words, what have marine sedimentary fauna done for you lately? Although I will focus on marine sedimentary environments, there are considerable parallels with freshwater sediments and terrestrial soils (e.g., Groffman and Bohlen 1999).
Global carbon and geochemical cycling. As a result of the global dominance of marine sedimentary habitats and the importance of sedimentary fauna in local carbon metabolism and burial through their feeding and mixing activities (e.g., Kristensen et al. 1992), sedimentary fauna influence global carbon dioxide dynamics and thus global warming. Other important geochemical cycles, such as those of sulfur and nitrogen, can also be affected by the organisms that reside in marine sediments. Marine sedimentary organisms of all sizes play a role in these processes. Bacteria, protozoa, and fungi are important decomposers and are thus important trophic links to larger organisms and nutrient cycling (see references in Snelgrove et al. 1997). Bacteria are also an important constituent of the diet of deposit feeders, along with the detritus that microbes help to decompose (e.g. Lopez and Levinton 1987). Macro- and megafauna, because of their large size, are particularly important in redistributing sediments and organic matter associated with sediments (see Gallagher and Keay 1998), thus affecting nutrient availability to different bacterial groups. Thus, the linkages between different sedimentary organisms are complex and their impacts on global cycling processes may be determined via direct and indirect routes. There are also multiple linkages between marine, terrestrial (Wall and Moore 1999), and freshwater (Covich et al. 1999) systems through these cycles.
Secondary production (food). Some sedimentary macrofaunal organisms are commercially fished (e.g., lobster, clams, and scallops) and therefore provide an important source of nutrition and employment for human populations. Macrofauna and meiofauna can also be major dietary components for commercial species, such as cod, shrimp, and flounder, that feed on benthos either as juveniles or as adults (e.g., Carlson et al. 1997). Thus, benthic organisms are an important part of the food chain and also transfer organic carbon back to the pelagic realm.
Pollutant metabolism and burial. Just as pollutants have a tremendous impact on soft-sediment benthos, these fauna also affect pollutant concentration and distribution. By pelletizing sediment as feces or stabilizing them through mucous excretion, organisms within the sediment can increase or decrease the likelihood of sediment-bound pollutants being re-suspended and transported elsewhere. Vertical mixing by sedimentary macrofauna can also increase or decrease the likelihood of burial, depending on whether animals feed at the surface or at depth (Gallagher and Keay 1998). If pollutants are bound to organic particles, then feeding activity may lead to their removal through incorporation into tissue; as a result, concentrations in the water column are reduced but the likelihood of transfer to higher trophic levels is increased if predators such as fish consume contaminated benthos. Microbes play a key role in the metabolic breakdown of pollutants and are consequently used in many waste treatment facilities. Because meiofauna play an important role in lower food webs, they also influence the fate of pollutants.
Filtration. Marine sedimentary habitats contribute to water clarity and health in several ways. Transition zones, such as marshes, seagrass beds, and mangroves act as sediment traps and stabilizers and buffer nutrient loading into the open ocean. Suspension feeders can have a major effect on water clarity through their filtering activity; the reduction of oysters in Chesapeake Bay through over-fishing, disease, and sedimentation has lowered filtering capacity and reduced water clarity (Newell 1988). The reverse problem has occurred in San Francisco Bay, where the introduced suspension-feeding Chinese clam, Potamocorbula amurensis, has attained sufficient densities to effectively strip the water of phytoplank-ton and eliminate natural seasonal blooms (Alpine and Cloern 1992). Thus, sedimentary communities contribute to ecosystem health not only within sediments but also in the water column above.
Sediment stability and transport. Sediment erosion and cohesion depend strongly on resident animals and microbes. Reworking by deposit feeders can substantially increase water content and erodibility (Rhoads 1974), and diatom films and mucus excretion can bind sediments and reduce erodibility. Physical structures, such as seagrasses, salt marshes, and mangroves also reduce erosion by trapping sediments. Thus, coastal zone communities can directly affect human environments by influencing coastal erosion (implications for land use) and deposition (implications for dredging of waterways).
An obvious question is whether marine sedimentary ecosystems can sustain loss of biological and genetic diversity and still provide the same sorts of ecosystem services that they have provided historically. The answer is yes and no. There are probably species that can be lost from some ecosystems without substantial alteration of system function. Two species often overlap in the way in which they feed, mix sediments, and decompose material (e.g., Whitlatch 1980). However, they probably do not carry out these activities in exactly the same way, and the functional significance of these differences probably depends on the species and ecosystem in question. In addition, there are some species whose loss will undoubtedly have serious direct or indirect consequences. The problem is that it is rarely known whether a given species or group of species is “critical,” making reduction of biodiversity a dangerous practice with potentially dire consequences.
Threats to marine sedimentary biodiversity
Although documented marine extinctions are rare, there is good reason to be concerned that marine biodiversity may be threatened. Because our knowledge of marine systems is so poor, it is likely that species are being lost without our knowing it. Although the lack of dispersal barriers in many marine areas might seem to reduce the odds of extinctions, I have already mentioned that species previously regarded as “cosmopolitan” may actually be species complexes (e.g., Grassle and Grassle 1976). In such cases, elimination of species from a given area could mean global extinction if “conspecifics” living elsewhere are actually sibling species. Moreover, genetic diversity may be lost as distributions shrink (e.g., Battaglia et al. 1980).
Fishing gear that is dragged across the bottom has destructive impacts on sedimentary fauna both directly and indirectly. The photos (from Auster 1998 and used with permission of Conservation Biology) show an area of the Gulf of Maine ocean bottom before and after a single pass with a scallop dredge. The pretrawled photograph (top) shows a complex bottom with polychaete tubes, sponges, and other forms of life. Structures of this sort also create habitat for other species. After the trawl has been dragged across the bottom, this complex habitat is obliterated, resulting in loss of habitat for animals that live within the structure (e.g., small Crustacea and fish), destruction of the fauna that creates the structure (polychaetes and sponges), and exposure, injury, or death of animals living within the sediment. These effects will likely influence all of the ecosystem services that benthic organisms provide.
Fishing activity affects sedimentary fauna most directly by dragging trawls and dredges across the bottom, physically damaging animals and destroying critical habitat for a variety of species that use the habitat structure created by epifaunal and infaunal presence and activity (see box this page; Botsford et al. 1997, Auster 1998). A second concern is the deliberate, large-scale removal of abundant and large predators (the target species), with coincidental bycatch of nontarget species, both of which can alter food chains and related ecological processes (Pauly et al. 1998). Fishing activity can also redistribute sediments, cause significant sediment resuspension, and alter sediment stability by disturbing species that influence cohesion and grain size (e.g., Rhoads 1974).
Dense human populations living near coastlines discharge large amounts of sewage, agricultural runoff, and toxic compounds, such as heavy metals and PCBs. Areas affected by these sorts of pollutants have high densities and low species diversity (Pearson and Rosenberg 1978). Genetic effects, such as a reduction in heterozygosity (e.g., Battaglia et al. 1980), can also be observed. Agricultural runoff and sewage outfalls provide excess nutrients that create both toxic and nontoxic algal blooms, which sink, decompose, and create anoxic conditions similar to those observed in freshwater systems (Covich et al. 1999). Coastal eu-trophication has also been linked to “red tides” that lead to paralytic shellfish poisoning in humans who ingest affected bivalves. Toxic compounds can be lethal to benthic organisms or may lead to reduced disease resistance or reproductive potential. Benthic organisms that are consumed by humans may also concentrate toxins (e.g., Lobel et al. 1990) that render them unfit for consumption. All of these disturbances reduce diversity and create a fauna of a few species that may be aesthetically undesirable and may not carry out ecosystem functioning the way a more diverse community would.
Physical alteration of habitat is a frequent result of agriculture and deforestation because increased sedimentation alters coastal sediment composition and thus the sedimentary biota (Smith and Kukert 1996). Beach replenishment, harbor dredging, and disposal of dredged sediments all have similar effects. The damming of estuaries can lead to changes in estuarine salinity gradients, sedimentation patterns, and biology. Perhaps the most damaging human alteration of marine sedimentary habitat is the filling of coastal wetlands. Saltmarshes, seagrass beds, and mangroves provide important ecosystem services, yet they have all suffered tremendous areal loss from coastal development.
Although oceans are interconnected, the presence of land masses, deep ocean, and differences in water temperature have created natural dispersal barriers and have thus allowed markedly different faunas to evolve in different areas of the world. Human activity is now wreaking havoc with these patterns. The greatest culprits are ship ballast water and ship hulls, which transport living adults and reproductive propagules to non-native habitats (e.g., Carlton 1985). Another mechanism of introduction is the accidental release of animals or their parasites after importation for aquaculture or scientific study. In many cases, exotic species explode in numbers and dominate to the detriment of native species, which may shrink in distribution or disappear from affected areas.
Given that temperature is a key delimiter of benthic distribution, it is likely that sedimentary faunal shifts have occurred, or will occur, as a result of global warming. Ultimately, global warming will compress or eliminate habitats as the fauna are shifted. A similar effect may be anticipated from rising sea levels as polar icecaps are reduced. Another concern is that global warming may change ocean circulation (Manabe et al. 1994), thus affecting productivity, larval and sediment transport, and ultimately the sedimentary community. Finally, ultraviolet radiation increases associated with ozone depletion could have direct impacts on shallow-water fauna and on the eggs and microplankton of organisms living in deeper water.
What do scientists need to do?
Given the present level of knowledge on biodiversity in marine sediments and how it influences ecological processes, what are the next steps that marine scientists should take if biodiversity is to be preserved?
Study processes and linkages in marine systems with respect to biodiversity and ecosystem functioning. Although many scientists believe that there is a relationship between biodiversity and how ecosystems operate, the lack of mechanistic detail in the examples cited above illustrates the need for specific and well-documented marine examples. In particular, studies that make these linkages in economically valuable marine systems are likely to have the greatest impact on legislation and conservation.
Recognize and promote taxonomy as an important scientific activity. Trying to document biodiversity pattern and stemming its loss will be impossible if retiring taxonomists are not replaced and more taxonomists are not trained.
Promote the concept of marine reserves, not only for areas deemed to be ecologically unique but also for areas that are representative of broader regions. These areas could be either partially restricted or completely closed to outside activities, as long as they achieve the critical need of providing natural ecosystem processes and preserving biodiversity. Scientists must provide the best possible information to identify areas that will provide the most important refuges, not only in terms of resident species but also for their capacity to enhance adjacent, nonprotected areas.
Scientists who have direct input on fisheries decisions should recommend quotas that are sufficiently conservative to ensure that any uncertainties in the models used to determine catch quotas cannot result in over-fishing in the worst-case scenarios. Recommendations on fishing gear should also consider, as a guiding principle, reducing bycatch, and subsidies to an overcapitalized fishing industry should be discouraged.
Every effort should be made to communicate to the general public the threats posed to marine sedimentary environments by destructive fishing practices, introduction of exotic species, pollution, destruction of natural coastline, and global climate change.
Because 1998 was the United Nations International Year of the Ocean, the timing is perfect for marine scientists to voice opinions and to communicate new findings on biodiversity in marine sediments. That designation for 1998 has helped to focus attention on oceans at a time when attention and changed perceptions are needed. Research on ecosystem operation and biodiversity is likely to result in exciting and far-reaching discoveries for sedimentary ecosystems. With some luck, it may be possible to change the tide of public perception of the oceans as an inexhaustible food resource and an environment too large and remote to be affected by the expanding sphere of human impact. Perhaps they will be seen instead as a vast storehouse for biodiversity that provides key ecosystem services.
Acknowledgments
I wish to thank J. P. Grassle, J. F. Grassle, M. Palmer, D. Wall, and three anonymous reviewers for suggestions that substantially improved the manuscript. Discussions with C. A. Butman were instrumental in beginning this synthesis. This article is a project of the Committee on Soil and Sediment Biodiversity and Ecosystem Functioning, a component of DIVERSITAS, coordinated by SCOPE.
References cited
Author notes
An associate chair of Fisheries Conservation in the Fisheries and Marine Institute, Memorial University of Newfoundland, Box 4920, St. John's, Newfoundland, Canada AlC 5R3. E-mail: psnelgro@gill.ifmt.nf.ca