A peptic ulcer is a chronic infectious disease that creates erosion on the epithelial lining of the stomach. It is a commonly encountered problem in the gastrointestinal tract (GI tract). Patients failed multiple regimens due to resistant H. pylori infection. H. pylori show maximum resistance towards Clarithromycin. Due to the consistent increase in resistance there is urgent need for the development of new drugs. Paying much for the antibiotic treatment one should go for the natural treatments with no side effects. Modern treatment of peptic ulcers emphasizes diet with routinely recommend hospitalization of several weeks. Currently, a lot of medications are coming out of natural products. The phytotherapeutic approach for the resistant H.pylori treatment is assessed. The plant produces many secondary metabolic substances which have a lot of beneficial roles in maintaining human health. Administration of plant products would prevent disease and able to eradicate resistant H.pylori. This review includes many phytoproducts having a wide range of antimicrobial activity. Reviewed phytoproducts includes Phytoceutical, Caffeic acid, phenethyl ester, Flavonoid, Capsaicin, Carotenoid, etc are effective treatment against H.pylori. To see their effect on the resistant H.pylori and to manage this resistant bacteria with an application of plant products is the prime concern of this review. The linkage between phytochemical and peptic ulcers will provide a novel framework for the future.
H. pylori, Peptic Ulcer, Plant Products, Resistant Antibiotics, Phototherapeutic Management, Ethnomedicine
The stomach is a glandular ‘J’ shaped organ lined with epithelial cells and it has a considerable amount of mucus mass and is constantly influenced by digestive enzymes, hydrochloric acid, microbes, and bile which in turn encounter producing mucus, prostaglandins, and bicarbonate to maintain the integrity of its lining, and this equilibrium is persistently synchronized and called mucosal defense. A peptic ulcer is due to an imbalance between mucosal protective mechanisms against damaging forces.1 It can be defined as the roughened area or cavity which is found in the stomach. Due to the hypersecretion of acid, it attacks the epithelial lining of the stomach which in turn causes soreness and is termed an ulcer. The ulcer is of two types that is a duodenal ulcer and gastric ulcer and combinedly termed as peptic ulcer.
The human body is exposed to numerous microorganisms in the environment every day. These organisms play important role in both health and diseases. H.pylori has been the main focus of study for gastrointestinal tract diseases for a long. It is a Gram-negative, microaerophilic organism that is associated with a variety of diseases like chronic gastritis, peptic ulcer, non-cardio gastric adenocarcinoma, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma.2,3 However, it’s important to understand at the outset that the vast majority of people who were infected with H. pylori when they are young are infected for life, and about three-quarters of these people or more have no symptoms in their whole lifetime. They would never know they had H. pylori unless somebody either cultures them or does another kind of test. A subset of people who have H. pylori develops gastritis. Some of these people go on to develop ulcer disease.
Duodenal ulcers and 70% of gastric ulcers are due to H. pylori infection.4 H. pylori-infected persons develop gastric damage. Among those infected patients, only 17% develop peptic ulcers.5 Triple therapy and quadruple therapy are used for the eradication of H. pylori but the rate of efficacy varies between 75% – 95% and the main reason for failure is due to antibiotic resistance.6
Despite several drug treatments that have been developed for various types of ulcer diseases, it has pernicious side effects. Emerging more resistant H. pylori is a worldwide problem and it demands the replacement of antibiotics with natural products. We believe that a combination of natural plant products that have anti-H. pylori activity could give the best result. This review was carried out with the help of bibliographic databases such as Google scholar, web of science, and PubMed.
Epidemiology and Prevalence of Peptic Ulcer
The rate of prevalence of H.pylori in developing countries is very high, that is about 80-90% in the case of adults in comparison with developed countries which is about 10-50% and this is due to low socioeconomic conditions.7 H. pylori infection is very common and in fact, is one of the most common infections worldwide. The rate of peptic ulcers brings down from before, but it has become more powerful than before and the occurrence of this disease are linked with age, sex, geographic environmental factors, and occupation.8,9 the frequency of peptic ulcers is between 19.4 – 57.0 per 100,000 people, with the number increasing every year.10
According to a study of 1335 peptic ulcer patients in Iran, the prevalence of peptic ulcers differed between male and female patients, with 60% and 30% respectively, and 62% of patients developed peptic ulcers as a result of H. pylori colonization and 30% as a result of environmental variables.11
Major Factors
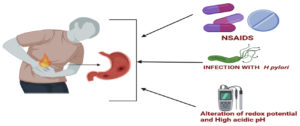
The first most common cause of peptic ulcer is infection with H. pylori and the second major cause of peptic ulcer is excessive secretion of hydrochloric acid (HCl). Higher concentration and higher volume of HCl secretion can alter the protective epithelial lining of the stomach and gastric cells because the amount is more than what the body can resist.8 The third cause is anti-inflammatory drugs like aspirin [Figure 1]. Studies showed that these drugs irritate the lining of the stomach and impair the barrier properties of the mucosa and increase the suppression of anti-inflammatory gastric prostaglandins, reducing gastric mucosa blood flow which affects the defense and repair of the lining of the stomach.12,13
Helicobacter pylori Pathogenesis
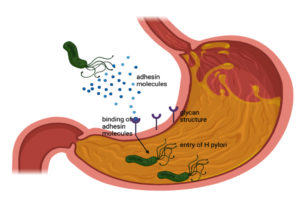
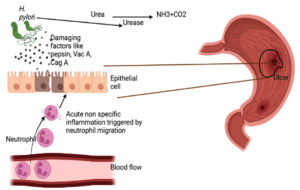
The most common cause of peptic ulcer is infection with H. pylori. It is a gram-negative, spiral-shaped bacteria having flagella that help in motility. The root of transmission is by fecal-oral route or through oral-oral route.14 The important virulence factor that H. pylori possess includes lipopolysaccharide which helps in adhering the cells, so it can attach itself to the gastric cells of the stomach by secreting adhesion molecule which is recognized by glycan structure that is expressed on the surface of gastric epithelial cells and the surface of mucus layer lining (Figure 2). Another important virulence factor of H. pylori is the enzyme on the surface known as urease and this enzyme protects from acidity as urease converts urea to ammonia and CO2. Further ammonia helps the bacteria from acidity by increasing pH to neutral as it is alkaline.15 This bacteria also secrete some exotoxins such as Vac A and Cag A. Vac A is a fairly potent kind of poison that causes apoptosis of cells and Cag A is responsible for disrupting cellular integrity and structure, and promotes inflammation. Cag A stimulates the production of certain chemokines such as IL-8 within its cells which in turn attracts neutrophils into the area. Neutrophils are highly inflammatory and can damage the stomach tissues (Figure 3). The combination of Cag A and Vac A causes stomach cells to break down, resulting in ulcer formation.16
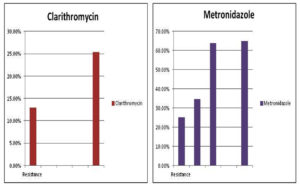
According to Khademi et al.21 the generality of resistance for clarithromycin is excessive than other antibiotics and that is about 64.9% which has been shown on the graph [Figure 4] [Table 1]. The prevalence of resistance for amoxicillin shows very little [Table 1].17-19 Our diet is rich in red meat, junk food, sugar, starch, etc. We do not consume the number of vegetables and fruits that we are supposed to take, and do not intake the right balance of foods that balance out our body systems and therefore end up with a lot of different diseases. A lot of natural supplements do help in regulating our bodies. Table 1. Illustrates the resistance of different antibiotics in different years.
Table (1):
Antibiotics preferred for the management of H. pylori.
| Antibiotics | Metronidazole | Clarithromycin | Amoxicillin | Tetracycline | levofloxacin | Reference |
|---|---|---|---|---|---|---|
| Resistance | 25.1% | 12.9% | 0.9% | 0% | [17) | |
| 34.7% | 16.7% | 11.8% | 18 | |||
| 63.9% | 37.2% | 0.3% | 1.2% | 50.3% | 19 | |
| 30%-40% | 65%-75% | 37.5% | 20 | |||
| 64.9% | 25.3% | 20.7% | 16.1% | 21.9% | 21 |
Polyphenol
Polyphenol is a bioactive secondary metabolic compound, generally obtained from fruits and vegetables, can suppress the negative effects of H. pylori in the GI tract. Traditionally, it is used for prevention of many diseases like cancer, neurodegenerative, osteoporosis, etc. It is potentially involved in inhibiting telomerase, cycloxygenase enzymes.32,33
Capsaicin
Capsaicin is a natural compound, extracted mostly from chili pepper shows a wide range of significant effects in maintaining the GI- tract. It shows cytoprotective effect, antioxidant property, anti-inflammatory, anti-proliferative effects, and inhibitory effect at 50ug/ml. Cancer cell line like MKN-45 in the stomach induces the production of IL-8, which is ultimately suppressed by administration of capsaicin. It can suppress the activation of NF-kB, whose activation is induced by H. pylori infection.34,35
Caffeic Acid Phenethyl Ester
Caffeic acid phenethyl ester are extracted products of propolis, have antioxidant, anti-carcinogenic, immunomodulatory, anti-inflammatory properties.23
Flavonoids
Flavonoids are the main constituents of most herbal products. According to Toshio fukai et al. Flavonoids (licoricone, gancaonol , vestitol and 1-methoxyphaseollidin) extracted from Glycyrrhiza glabra is a potential herbal medicine against resistance H. pylori as it shows inhibitory effect on both amoxillin and clarithromycin resistance strain.36 In stress condition, flavonoids are released from plants as secondary metabolic polyphenolic substances and protect the plant from a pathogen.37
Quercetin
Quercetin is found broadly in most vegetables and fruits and has strong antioxidant properties. The skeletal structure of Quercetin is quite similar to flavone. According to Xin-Ting et al. gastroprotective mechanism has been observed with the application of Quercetin. When Quercetin is applied on gastro epithelial cells, it is observed that loss of cell viability due to hydrogen peroxide, reduces drastically. It is also found that it suppresses Calcium ion influx and reactive oxygen species activity.38
Curcumin
It has anti-oxidant, anti-inflammatory, anticarcinogenic, and antimicrobial properties. It inhibits iNOS, COX-2, LOX, mediated signaling pathways. It reduces the inflammation which is caused by certain cytokines like TNF-a, IL-6, and IL-1β.39,40
Carotenoids
Carotenoids can act more efficiently than metronidazole. The minimum inhibitory concentration of carotenoids is considerably high than the amoxicillin and clarithromycin but Carotenoids can act more efficiently than metronidazole. Carotenoids like neoxanthin and luteoxanthin show more effective results than other carotenoids.31
Ethanol Extract
It is observed that the application of garlic can give a significant result. About 1-4% of garlic intake can suppress the gastric lesion in H. pylori-infected individuals.
It can be used for various pharmacological functions like wound healing, anti-cancer, anti-oxidant, immunomodulatory potential, anti-inflammatory, etc. It inhibits the growth of different strains of bacteria and fungi. Allicin and ethanol bioactive chemicals present in garlic make it more potent against various kinds of diseases.41
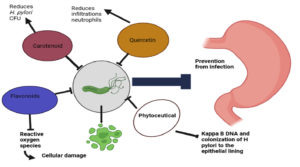
Table 2 Emphasizes the plant products having a wide range of antimicrobial properties. Phytoceutical which is obtained from broccoli, garlic, green tea, etc. And polyphenol from apple peel can be used in the management of H.pylori products.
Table (2):
List of plant products having anti-H. pylori activity.
Sl. No. |
Plant products |
Source |
Role |
Reference |
|---|---|---|---|---|
1 |
Capsaicin |
Chilies |
Decreases IL-8 production |
22 |
2 |
Caffeic acid phenethyl ester |
Propolis |
Suppresses inflammation and also decreases the density of H. Pylori |
23 |
3 |
Flavonoid |
Sideritis mugronensis |
Increases prostaglandins which in turn boosts defense mechanism in the stomach |
24(Fig.5) |
4 |
Acacetin and luteolin |
Chamomile tea, broccoli, carrots, apple skin |
Reduces ulcer which happens due to histamine induction. |
24 |
5 |
Phytoceutical |
Probiotics, Green tea, broccoli sprouts, red wine, flavonoids, garlic, |
It prevents the colonization of H. pylori to the epithelial lining It also halts cancer by Suppressing the activity of kappa B DNA |
25 (Fig.5) |
6 |
Flavonoids and proanthocyanidins |
Citrus fruits, berries, onions, and legumes |
Due to antioxidant properties, it prevents reactive oxygen species which causes cellular damage |
26 (Fig.5) |
7 |
Quercetin |
Vegetables and fruits |
Reduces neutrophils |
27(Fig.5) |
8 |
polyphenol |
Apple peel |
Anti-inflammatory and reduces colonization of H. Pylori |
28 |
9 |
Ethanol extract |
Garlic |
Reduces erosion on the stomach |
29 |
10 |
Curcumin |
Turmeric plant |
Plays vital role in the eradication of H. Pylori |
30 |
11 |
Carotenoid |
Apple peel |
Reduces H. Pylori CFU |
31 (Fig.5) |
Many plants having anti-ulcer and other medicinal properties were used as ethnomedicine worldwide. It is obtained from the data that Phytoproducts like Phytoceutical, flavonoids, and carotenoids, protect the lining of the stomach by preventing colonization of H. pylori and also has erosion healing activity. Its antioxidant property provides defense towards reactive oxygen species. Further, more clinical trial is needed concerning the following points-
- Standardization of plant products is necessary to deal with peptic ulcers.
- We can normalize Eh and the pH of the stomach using Phytoproducts.
A comparative study should be carried out with a large sample size on different populations with different diets to rule out the efficacy of plant products in the management of H. Pylori induced peptic ulcers.
ACKNOWLEDGMENTS
The authors would like to thank President of Siksha ‘O’ Anusandhan deemed to be University, India for providing necessary facilities to carry out this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RB conceptualized and designed the work. SL perform the literature survey and wrote the manuscript. RB reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Yandrapu H, Sarosiek J. Protective Factors of the Gastric and Duodenal Mucosa: An Overview. Curr Gastroenterol Rep. 2015;17(6):24.
Crossref - Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420-429.
Crossref - Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863-1873.
Crossref - Sverden E, Agreus L, Dunn JM, Lagergren J. Peptic ulcer disease. BMJ. 2019;367:l5495.
Crossref - Malaty HM, Nyren O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2003;8(Suppl 1):8-12.
Crossref - Graham DY, Qureshi WA. Antibiotic-resistant H. pylori infection and its treatment. Curr Pharm Des. 2000;6(15):1537-1544.
Crossref - Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect. 2003;5(8):693-703.
Crossref - Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390(10094):613-624.
Crossref - Malfertheiner P, Schulz C. Peptic Ulcer:Chapter Closed? Dig Dis. 2020;38:112-116.
Crossref - Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102-113.
Crossref - Sayehmiri K, Abangah G, Kalvandi G, Tavan H, Aazami S. Prevalence of peptic ulcer in Iran: Systematic review and meta-analysis methods. J Res Med Sci. 2018;23:8.
Crossref - Konturek SJ, Piastucki I, Brzozowski T, et al. Role of prostaglandins in the formation of aspirin-induced gastric ulcers. Gastroenterology. 1981;80(1):4-9.
Crossref - Rout P, Pradhan D, Behera PK. Complication, pathogenesis and medication of perforated peptic ulcer: a paradigm shift in today’s treatment. World Journal of Pharmaceutical Research. 2018;7(9):275-287.
Crossref - Kitchens DH, Binkley CJ, Wallace DL, Darling D. Helicobacter pylori infection in people who are intellectually and developmentally disabled: a review. Spec Care Dentist. 2007;27(4):127-133.
Crossref - De Falco M, Lucariello A, Iaquinto S, Esposito V, Guerra G, De Luca A. Molecular Mechanisms of Helicobacter pylori Pathogenesis. J Cell Physiol. 2015;230(8):1702-1707.
Crossref - Abu-Taleb AMF, Abdelattef RS, Abdel-Hady AA, et al. Prevalence of Helicobacter pylori cagA and iceA Genes and Their Association with Gastrointestinal Diseases. Int J Microbiol. 2018;2018:4809093.
Crossref - Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10(6):1088-1094.
Crossref - Bang SY, Han DS, Eun CS, et al. Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease. The Korean Journal of Gastroenterology=Taehan Sohwagi Hakhoe chi. 2007;50(6):356-362. https://europepmc.org/article/med/18159172
- Akhtereeva AR, Morozova LG, Faizullina RA, et al. Antibiotic susceptibility assessment of Helicobacter pylori isolates by disk-diffusion method. BioNanoScience.2018;8(3):930-934.
Crossref - Wu IT, Chuah SK, Lee CH, et al. Five-year sequential changes in secondary antibiotic resistance of Helicobacter pylori in Taiwan. World J Gastroenterol. 2015;21(37):10669-10674.
Crossref - Khademi F, Sahebkar A. An Updated Systematic Review and Meta-Analysis on the Helicobacter pylori Antibiotic Resistance in Iran (2010-2020). Microb Drug Resist. 2020;26(10):1186-1194.
Crossref - Lee IO, Lee KH, Pyo JH, Kim JH, Choi YJ, Lee YC. Anti-inflammatory effect of capsaicin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2007;12(5):510-517.
Crossref - Abdel-Latif MM, Windle HJ, Homasany BS, Sabra K, Kelleher D. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br J Pharmacol. 2005;146(8):1139-1147.
Crossref - Lewis DA, Hanson PJ. Anti-ulcer drugs of plant origin. Prog Med Chem. 1991;28:201-231.
Crossref - Lee SY, Shin YW, Hahm KB. Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection. J Dig Dis. 2008;9(3):129-139.
Crossref - Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124.
Crossref - Gonzalez-Segovia R, Quintanar JL, Salinas E, Ceballos-Salazar R, Aviles-Jimenez F, Torres-Lopez J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J Gastroenterol. 2008;43(6):441-447.
Crossref - Farzaei MH, Abdollahi M, Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol. 2015;21(21):6499-6517.
Crossref - Iimuro M, Shibata H, Kawamori T, et al. Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett. 2002;187(1-2):61-68.
Crossref - De R, Kundu P, Swarnakar S, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53(4):1592-1597.
Crossref - Molnar P, Deli J, Tanaka T, et al. Carotenoids with anti-Helicobacter pylori activity from Golden delicious apple. Phytother Res. 2010;24(5):644-648.
Crossref - Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113(4):660-669.
Crossref - Naasani I, Oh-Hashi F, Oh-Hara T, et al. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63(4):824-830.
- Jones NL, Shabib S, Sherman PM. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol Lett. 1997;146(2):223-227.
Crossref - Lee KH, Lee YC, Kim TI, et al. Inhibitory effect of capsaicin on interleukin-8 production by Helicobacter pylori-infected MKN-45 cells. J Microbiol Biotechnol. 2006;16(7):1078-1083.
- Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71(12):1449-1463.
Crossref - Nabavi SM, Samec D, Tomczyk M, et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol Adv. 2020;38:107316.
Crossref - Hu XT, Ding C, Zhou N, Xu C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur J Pharmacol. 2015;754:115-124.
Crossref - Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141-153.
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105-125.
Crossref - Londhe VP, Gavasane AT, Nipate SS, Bandawane DD, Chaudhari PD. Role of garlic (Allium sativum) in various diseases: An overview. Angiogenesis. 2011;12:13:129-134.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.