ISSN: 0973-7510
E-ISSN: 2581-690X
Trachyspermum ammi seeds were selected for photochemical study. The crude T. ammi methanol and aqueous extracts showed the presence of alkaloids, saponins, steroids, terpenoids, coumarins, betacyanins, flavonoids and soluble starch. The synthesis of gold nanoparticles (AuNPs) using T. ammi extract was characterized using UV-visible, and FT-IR spectroscopy. The appearance of sharp peak at 520 nm in the UV visible spectra, and the appearance of broad band nanoparticles spectra at 563.21 cm-1, 516.92 cm-1 and 462.92 cm-1 as well as the disappearance of the carboxyl OH bond and carbon-carbon triple bond supported the formation of AuNPs. The crude T. ammi methanol and AuNPs were investigated for antioxidant potential using DPPH· free radical assay, which shows that crude extract has significant antioxidant effect. The synthesized AuNPs was also evaluated for antibacterial activities against staphylococcus aureus, Klebsiella pneumonia and Bacillus subtilis. The crude extract showed activity against Bacillus subtilis, while AuNPs showed activity against staphylococcus aureus. The in vivo sedative effect and analgesic effect were enhanced in AuNPs treated animals in 5 times less dose (i.e., 5, and 10 mg/kg) than that of crude extract. It was concluded that T. ammi extract includes capping and reducing agents, which make it capable to be developed as stable AuNPs. The biological action of AuNPs is either enhanced (sedative and analgesic) or changed (antibacterial activity), when compared with that of plant extract.
Nanotechnology, phytochemicals, biological activities, sedative effect, analgesic effect
The medicinal plants provided the ailments and cure throughout human history. Medicinal plants are e capable of making various ranges of secondary metabolites which possess diverse biological potentials1. Even at dawn of 21st century, about 90% of probable novel drug molecules have been purified directly or indirectly2. As per WHO report about 80% of the people of some Africo-Asian countries nowadays, use herbal medicine for some aspect of primary health care3. The clinical application of herbal drugs faces the same challenges as allopathic medicines like effectiveness, drug delivery, safety, selectivity, solubility, toxicity and frequent dosing4,5. The modern pharmaceutical research could overcome the abovementioned challenges by adopting novel drug delivery techniques for herbal medicines, such as development of matrix system, micro emulsion, solid dispersion, liposomes and solid lipid NPs, nanoparticles (NPs)6,7.
The field of nanotechnology has grown widely in past few decades with efficient applications in biomedical sciences, food processing, and pharmacology8-10. It deals with matter at the scale of one billionth of a meter, so the NPs is the most fundamental component in the fabrication of a nanostructure. The nano-size atoms and molecules exhibit different properties, and ultimately show a variety of surprising and interesting results11,12. There are various methods reported for fabrication of NPs, but metal NPs like gold and silver are extensively studied due to their unique electrical and optical properties. Gold nanoparticles (AuNPs) are increasingly studied because of their wide applications in antibacterial agents, detectors, and sensors13-15. In old Indian medical system (Ayurveda), gold salt was used as medicine in the preparations to treat anemia, tuberculosis, and cough. In traditional medicines, the gold has been explored for various disease ailment and shown with efficient antibacterial and antifungal properties16-19.
Trachyspermum ammi is an annual herbal plant belongs to family Apiaceae, indigenous to Egypt but cultivated in Iran, Pakistan and India and Europe. It is commonly known as Ajwain in India. The green seeds as well as fruits T. ammi are well recognized for medical and nutritional purposes20. The structural composition of T. ammi include proteins (17.1%), fats (21.1%), glycosides (12%), carbohydrates, saponins, flavones and other components (7.1%) involving copper, calcium, cobalt, iron, iodine, manganese, nicotinic acid, phosphorous, riboflavin, and thiamine. The T. ammi is known for its brownish essential oil (5%) that responsible for its odor and taste21. The plant has medical application in Medieval and Traditional Persian Medicine for paralysis, tremor and palsy as well as other disorders in the field of neurology. The plant used for the auditory and olfactory weakness and infections20,21. The T. ammi was beneficial in cough, Pleurisy and dysphonia, anthelmintic, gastrointestinal disorders and have carminative properties. The Persian practitioner considered the seeds as a diuretic agent and beneficial for dissolving the stones when used with wine22.
In the present study, we aimed to synthesize the AuNPs of T. ammi methanol and aqueous extract keeping in view its extensive medical applications. The synthesized AuNPs were characterized and analyzed for its stability. The newly synthesized AuNPs were biologically screened analgesic, sedative and antibacterial effects and compared with respective therapeutic effect of the crude T. ammi extract.
Chemicals and plant sampling
Gold chloride, potassium bromide, 1,1-diphenyl-2-picrylhydrazyl (DPPH·), methanol, sulphuric acid, and trichloro methane were from Sigma (St. Louis, MO, USA). Trachyspermum ammi was procured from local market (Peshawar, Pakistan). The plant was identified by taxonomist Ghulam Jilani from Botany Department, University of Peshawar, Pakistan.
Extraction of T. ammi seeds
To prepare the extract, 500 g of T. ammi seeds were dipped in distilled water and methanol using conical flask, respectively and kept in water bath. The two solutions, obtained after three days, were filtered and the filtrates were concentrated in rotary evaporator. Further evaporation was allowed in water bath for two days to procure water and methanol solid extract21,22.
Phytochemicals profiling
Tests for phytochemicals like tannins, anthraquinones, alkaloids, saponins, glycosides, reducing sugars, flavonoids, steroids, terpenoids, phlobatanins, anthocyanins, betacyanins, cardiac glycosides, emodins, coumarins and free reducing sugars were carried out20-22.
Synthesis of AuNPs
Preparation of stock and gold salt solution
The extract stock solution was prepared by dissolving 1 g of solid extracts of T. ammi (methanolic and aqueous) in 100 mL methanol and/or water. Another stock solution of gold salt was prepared by adding 34 mg of gold chloride to 100 mL of methanol solution18.
Formation of AuNPs
Four samples of 5 mL stock solution of water and methanol extracts were added to the 5 mL, 10 mL, 15 mL, and 20 mL gold salts solution separately, stirred on hot stirring plates on 40 to 60°C for 60 min and observed its UV spectrum for confirmation of synthesis of AuNPs18.
Characterization of AuNPs
UV visible spectroscopy
UV visible spectra was recorded at room temperature to monitor the formation and stability of AuNPs. The wave length (ʎ) were fixed between 300 nm and 700 nm and the base line was drawn by the methanol solution. The peak in the 500-600 nm range indicates the presence of AuNPs23.
FTIR Spectroscopy
An FTIR spectrum was recorded and the measurement was performed to recognize the possible functional groups of molecules responsible for capping and effective stabilization of the nanoparticles synthesized18.
Biological evaluation of crude extract and AuNPs
The crude extract and synthesized AuNPs were evaluated in vitro and in vivo for various biological activities.
In vitro biological activities
Antibacterial activity
Antibacterial activity was performed by disk diffusion technique. The procedure was done in laminar airflow, Forceps, Petri dish, cotton swab, disc sterilized and utilized for plating. Sterile environment was maintained by HEPA (high performance efficient particle air) in the laminar flow. A few colonies of the organism are inoculated in 5 mL broth and cultured for 2 h. The cultures were diluted to a density of the 1 % barium sulfate standard. After 15 min bacterial cultures were spread on agar surface by spread plating method. When the inoculum dried, the impregnated discs were spread on the agar surface with flamed forceps and pressed down to ensure contact. The discs were sterilized by autoclaving and then dried at 80°C for at least 1 h. The sterile discs were immersed in extract solution (2 mg/mL), and AuNPs solution (1 mg/mL) then placed on Petri plates. The impregnated discs are dried for 5 min and washed with water. The discs dried at 25°C for 15 min were pressed down to make certain complete contact of the disc with the agar surface. Ampicillin was used as positive control for antibacterial screening test. The discs were spread out far sufficient to stop both reflection waves from the preparation of methanolic extract edges of the Petri plates and overlapping rings of inhibition. The plates were incubated at 37°C for 12 h. The diameter of the zone of inhibition was determined19.
Antioxidant activity
The stock solution of crude extract was organized by dissolving 25 mg in 50 mL methanol. For antioxidant activity, different concentrations were prepared from stock solution by diluting with methanol (i.e., 20, 40, 60, 80, 100 and 150 µg/mL). Similar concentrations were prepared for AuNPs. One mL of DPPH· was added to each of PPM concentration and the solution was placed in the darkness for 30 min and then UV-visible spectra was measured at 515 nm. Antioxidant activity of both crude and nanoparticles base line is made on a solution of 4 mL methanol and 1 mL DPPH· and it’s percent activity is calculated by the following formula24:
Percent (%) activity = control-absorbance/control*100
In vivo biological activities
Animals
In this experiment BALB/c mice were used. Mice were kept under standard light/dark cycles and temperature with standard supply of food (normal laboratory food and water ad-libitum). Prior to the experiment, mice were familiarized with laboratory conditions. The rulings of the institutes of Laboratory Animal Resources, Commission on Life Sciences, National Research Council were maintained during whole course of study. The protocols of all experiments were approved by the ethical committee of the Department of Pharmacy, University of Peshawar, Pakistan.
Acute toxicity testing
The acute toxicity screening was done as per reported procedure of Khan et al. Animals were divided into various groups (n= 6). The extract of T. ammi was screened at 50, 100, 200, 300, 400, 500 mg/kg (body weight of mice). The AuNPs of T. ammi was screened at 1, 5, 10, 20, 30, 40 mg/kg (body weight mice). After administration (i.p.) of the above-mentioned test doses, the animals were observed for 24 h duration. The mortality and survival ratio was noted and percent mortality was calculated25.
Sedative activity
The design of the device for sedative activity covers an area of whitish woody structure possessing a diameter of 1500 mm. The stainless steel was used for fencing and the structure was partitioned into 19 square boxes. Open field of the structure was positioned in a room with ample space. Balb-C mice (n=6) weighing 26 ± 4 g were used. The mice were familiarized with light of red color by keeping them under a 40 Watt red bulb for 1 h prior to start of the experiment. The normal control animals were injected distilled water as blank (10 mL/kg). The reference group was treated with 0.5 mg/kg diazepam. The rest of the groups were treated with crude extract (50, and100 mg/kg, i.p.) and AuNPs (5 and 10 mg/kg, i.p). After a time interval of 30 min, all mice were placed one by one in the middle of box, and the number of lines passed by each animal was noted25.
Acetic acid induced writhing test
Peripheral nociceptive action of T. ammi extracts along with synthesized AuNPs was examined by applying the acetic acid-induced writhing test25. The pre-screened mice were distributed in 6 groups where each group contained six animals. The pain was triggered by injecting 0.9% acetic acid (v/v, 0.1 mL/10 g body weight) intraperitoneally. The control group (six animals) was given normal saline (10 mL/kg, i.p.), whereas second group was provided with diclofenac sodium (10 mg/kg i.p.) as a standard drug. The remaining groups received Trachyspermum ammi extract (50 and 100 mg/kg i.p) and synthesized AuNPs (5 and 10 mg/kg i.p). After the injection of 0.9% acetic acid, the total numbers of contractions in muscles were recorded for 20 minutes each group and compared to that of control group (saline treated group).
Statistical analysis
All of the results are presented as mean ± S.E.M and One-way ANOVA was applied in order to compare significant variances among groups followed by Dunnet’s multiple comparison post-test. The significance level (p < 0.05 or 0.01) was considered valid in the study. The results of antibacterial and antioxidant activities are also given as mean ± SEM of three readings and their statistical analysis were carried out using the GraphPad program.
Phytochemical screening
The analysis of T. ammi aqueous and methanol extract exhibited the presence of various phytochemicals (Table 1). The aqueous extract of T. ammi showed positive result for the presence of alkaloids, flavonoids, terpenoids, and saponins. Methanol crude extract showed positive results for the presence of flavonoids, betacynins, terpenoids, and steroids. This preliminary phytochemical screening made it clear that the both extracts have various phytochemicals which would act as both capping and reducing agents for the synthesis of NPs20-22.
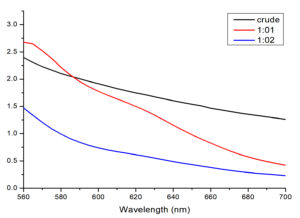
Fig. 1. UV-visible spectra of T. ammi extract solution and synthesized AuNPs
Where, Crude: extract solution of T. ammi, 1:01: showed AuNPs synthesized by mixing stock solution and salt solution (1:1, v/v),1:02: showed AuNPs synthesized by mixing stock solution and salt solution with (1:2, v/v)
Characterization of AuNPs
UV-visible spectroscopy
The production of AuNPs is generally detected by UV-Vis spectroscopy which specifically measures surface plasmon resonance peaks of AuNPs. The noble metals exhibited distinctive optical properties due to the property of surface plasmon resonance. When T. ammi extract was added to salt solution at 40-60°C temperature, the solutions changed from yellow to brown, indicating the formation of AuNPs. The color of the solution was changed because of reduction of gold (Au3+ to Au0) by the active phytochemicals present in T. ammi extract26. Previously reported studies showed that phytochemicals act as capping and reducing agents. The variety of constituents in the natural extracts leading to the synthesis of symmetrical NPs27. Experiment was carried out with varying stock solutions of T. ammi extract and salt concentrations. In order to monitor the formation and stability of AuNPs, the absorption spectra of the synthesized AuNPs were recorded against methanol. The peak absorbance of AuNPs was observed in wavelength (ʎ) range of 500-600 nm in methanol solutions, which exhibited the successful formation of AuNPs.
Table (1):
Phytochemical assortment of Trachyspermum ammi extracts.
Chemical components |
Aqueous extract |
Methanol extract |
|---|---|---|
Alkaloids |
+ |
– |
Tannins |
– |
– |
Anthraquinones |
– |
– |
Glycosides |
– |
– |
Reducing Sugars |
– |
– |
Saponins |
+ |
– |
Flavonoids |
+ |
+ |
Phlobatannins |
– |
– |
Steroids |
– |
+ |
Terpenoids |
+ |
+ |
Cardiac glycosides |
– |
– |
Coumarin |
– |
+ |
Emodins |
– |
– |
Betacyanins |
– |
+ |
Carbohydrates |
– |
– |
Monosaccharides |
– |
– |
Free reducing sugars |
– |
– |
Combined reducing sugars |
– |
– |
Starch |
– |
– |
FTIR spectra analysis of crude extract and AuNPs
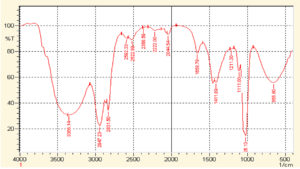
The FTIR spectra acquired from the crude extract of T. ammi and synthesized AuNPs are demonstrated in Fig. 2 and 3. In Fig. 2, The O-H bonds stretching is attributed by a broader peak observed at 3392 cm-1, evidenced the presence of aromatic alcoholic and phenolic compounds. The peaks such as 2947.23 cm-1, 2831.50 cm-1, 2592.33 cm-1, and 2522.89 cm-1 reflect carboxylic acid bond stretching. The peak at 2368.59 cm-1, 2222.00 cm-1 and 2044.54 cm-1 show C≡C stretching. The peak of 1658.78 cm-1 shows carbonyl group and peak at 1411.89 cm-1 shows C-C stretching. The peak at 1211.30 cm-1 shows C-N stretching and peak at 1026.13 cm-1 shows C-H single bond stretching. The peak at 655.80 cm-1 shows C-Br stretching.
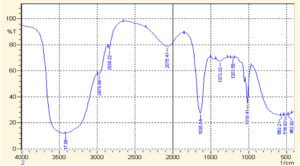
Fig. 3. FTIR spectrum of AuNPs synthesized by mixing extract stock solution and salt solution (1:2, v/v)
When this FTIR spectra was compared with that of AuNPs, the spectra exhibited different IR absorption. The FTIR spectra acquired for AuNPs after 1 hour of reaction for observing the involved functional groups present in T. ammi extract (Fig. 3). The peaks at 2592.33 cm-1, 2522.89 cm-1, 2368.59 cm-1 and 2222.00 cm-1 disappear in the spectra of FTIR spectra of AuNPs. Thus, it means that carboxyl bond and carbon-carbon triple are considered accountable for reduction and stabilization of AuNPs28. Broad bands observed in spectra of AuNPs 563.21 cm-1, 515.92 cm-1 and 462.92 cm-1 validated the formation of AuNPs, which were not observed in the crude FTIR Spectrum29.
Biological evaluation of crude extract and AuNPs
Antioxidant Activity
The free radical scavenging against DPPH· is a simple and commonly used assay for the study of antioxidant potential of tested compounds30-32. This is a chromophoric assay in which change in absorbance is indicated by change in color and then measured. The free scavenging effect of crude extract vs synthesized AuNPs is shown in the Table 2. The antioxidant activity of crude extract of T. ammi was higher as compared to the AuNPs. As the concentration of the stock solution and AuNPs increases, its antioxidant effect increases. The data demonstrated that the net antioxidant effect of AuNPs decrease as compared to extract.
Table (2):
DPPH• free radical scavenging activities of T. ammi crude extract and AuNPs
| Concentration (μg/mL) | Percent activity (%) | |
|---|---|---|
| Methanol extract | AuNPs | |
| 20 | 44.79 | 36.40 |
| 40 | 58.91 | 46.97 |
| 60 | 64.51 | 54.56 |
| 80 | 76.34 | 60.16 |
| 100 | 82.17 | 61.60 |
| 150 | 84.22 | 63.59 |
Antibacterial activity
To analyze the comparative antibacterial effect of crude extract and AuNPs, they were screened against gram positive and negative bacteria as given in Table 3. The crude extract as well as synthesized AuNPs were ineffective against Klebsiella pneumonia. The crude extract exhibited sample shows activity for Bacillus subtilis, while AuNPs showed activity for staphylococcus aureus. However in both cases the zone of inhibition (10mm) was far less than that of standard ampicillin (30mm). The data exhibited that the AuNPs synthesis of T. ammi extract changed its antibacterial properties. The thymol isolated from T.ammi is potent antibacterial agent and considered to disrupt bacterial membrane function34.
Table (3):
Antibacterial activity of T. ammi crude extract and AuNPs.
| Microorganism | Gram stain | Crude extract | AuNPs | Ampicillin |
|---|---|---|---|---|
| Diameter of zone of inhibition (mm) | ||||
| Klebsiella Pneumonia | – | 0 | 0 | 30 |
| Staphylococcus aureus | + | 0 | 10 | 30 |
| Bacillus subtilis | + | 10 | 0 | 30 |
Acute toxicity dose determination
The acute toxicity of T. ammi crude extract was assessed in dose of 50, 100, 200, 300, 400, and 500 mg/kg. Whereas the AuNPs was screened at doses of 1, 5, 10, 20, 30, and 40 mg/kg. The animals exhibited behavioral toxicity, no mortality during 24 h of assessment. For the comparison of in vivo effect assessment between extract and AuNPs, extract dose used was 50, and 100 mg/kg, however, AuNPs dose used was 5-times less than that of extract (i.e., 5 and 10 mg/kg).
Table (4):
Sedative activity of Trachyspermum ammi extract and AuNPs.
| Treatment | Dose (mg/kg) |
No. of lines crossed in 10 min |
|---|---|---|
| Distilled water | 10 | 125±1.15 |
| Diazepam | 0.5 | 6 ± 0.14 |
| Extract | 50 | 122.4±2.70 |
| 100 | 113.4 ±2.42 | |
| AuNPs nanoparticles | 5 | 96.56±0.84 |
| 10 | 82.40±1.78 |
Sedative activity
The sedative effect of natural products is frequently assessed by application of open field assay in experimental animals33. By comparing the treated (with extract and AuNPs) groups with diazepam-treated group, it was found that T. ammi has very high potential for sedation. Pretreatment of mice with T. ammi crude extract (50 mg/kg) showed locomotory activity comparable to normal animal, however, slight decrease was observed at dose of 100 mg/kg. Pretreatment of mice with AuNPs (5, and 10 mg/kg) showed locomotory activity comparable with that of high doses of extract (50, and 100 mg/kg). The data showed that the effect of AuNPs enhanced compared to T. ammi extract. The sedative potential of T. ammi has already been explored34. Various compounds have tendency to change neurological behavior35. The monoterpene (thymol) isolated from T. ammi is evaluated as allosteric modulator of GABAA receptor36. The same GABA receptor is the site of action for reference drug diazepam thereby increasing the chloride influx and causes neuronal hyperpolarization.
Table (5):
Analgesic activity of Trachyspermum ammi extract and AuNPs.
| Treatment | Dose (mg/kg) | No. of lines crossed in 10 min |
|---|---|---|
| Normal saline | 10 (mL/kg) | 65.4±3.48 |
| Diclofenac sodium | 1 | 17.6±1.38 |
| Extract | 50 | 52.3±2.44 |
| 100 | 30.2±2.13 | |
| AuNPs | 5 | 48.2±2.00 |
| 10 | 26.3±1.67 |
Analgesic activity
Various methods are applied for analyzing the peripheral or central analgesic potential of plant extracts and drugs. One of most preferred method for assessment of peripheral analgesia is the acetic acid induced pain model, due to its sensitivity, rapid execution37. The method is based on the principle of analyzing abdominal writhes (constriction) produced by inflammatory markers after acetic acid injection. The substances producing inhibition of writhing may associate with decline production or inhibition of inflammatory markers, such as prostaglandins, prostacyclins, in the decreased production or inhibition of proteinoids, at peripheral level38,39. The substances capable of inhibition of receptors for the inflammatory markers could exhibit the peripheral analgesic effect. The analgesic effect of active constituents present in samples under analysis is measured in terms of inhibition of acetic acid induced abdominal constrictions (writhing). Therefore, we evaluated the analgesic potential of extract and AuNPs by using acetic acid induced writhing test. Our data showed that T. ammi extract in 50 mg/kg has mild analgesic potential, which increased to moderate effect at dose of 100 mg/kg compared to reference drug. Slight enhanced analgesic activity was observed in less doses of AuNPs (5 to 10 mg/kg). The analgesic and anti-inflammatory potential of T. ammi has already been explored in various studies34,40. The T. ammi extract contain terpenes, sterols and glycosides, which are suggested to have effect on kinin, bradykinin, prostaglandins and lysozymes synthesis thereby reducing inflammation and allodyna (pain)41,42.
The data concluded that the T. ammi extract have necessary capping and reducing agents, which make it capable to be developed as stable AuNPs. The AuNPs showed enhanced biological effect which explain that the pharmacokinetic parameters like bioavailability is increased. The biological action of AuNPs is enhanced in case of antibacterial activity, which means that AuNPs might change the specificity of T. ammi and likewise other drugs.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research group Project under grant number (R.G.P. /52/42). The work is funded by grant number 14-MED333-10 from the National Science, Technology and Innovation Plan (MAARIFAH), the King Abdul-Aziz City for Science and Technology (KACST), Kingdom of Saudi Arabia. We thank the Science and Technology Unit at Umm Al-Qura University for their continued logistic support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SB, AR, HN, SUAS, JA, GU, and F drafted the manuscript, compiled information from the literature, and designed the Fig. and tables. SB, AR, HN, SUAS, JA, GU, and F drafted the manuscript and gathered information from the literature. MFR reviewed the manuscript.
FUNDING
Deanship of Scientific Research at King Khalid University for funding this work through Research group Project under grant number (R.G.P. /52/42).
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Daniel AD, Sylvia U, Ute R. A Historical Overview of Natural Products in Drug Discovery. Metabolites. 2012;2(2):303-336.
Crossref - Veeresham C. Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3(4):200-201.
Crossref - Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Persp. 2001;109(1):69-75.
Crossref - Yadev D, Suri S, Choudhary AA, et al. Novel approach, herbal remedies and natural products in pharmaceutical science as nano drug delivery systems. Int J Pharm Tech Res. 2011;3(3):3092-3116.
- Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: a pharmaceutical prospective. J Control Release. 2006;113:189-207.
Crossref - Kumar K, Rai AK. Miraculous therapeutic effects of herbal drugs using novel drug delivery systems. International Research Journal of Pharmacy. 2012;3:27- 30.
- Sharma AT, Mitkare SS, Moon RS. Multicomponent Herbal Therapy: A Review. International Journal of Pharmaceutical Sciences Review and Research. 2011;6:185-187.
- Rajiv S, Santosh S, Sharma, S. Nanotechnology: the future medicine. J. Cutan. Aesthet. Surg., 2010, 3, (1), pp. 32-33.
- Bawazeer S, Rauf A, Rahman KU, et al. Green Synthesis and Antimicrobial Potential of Silver/Gold Nanoparticles Functionalized with Debregeasia salicifolia D. Don. J Pure Appl Microbiol. 2020;14(4):2513-2523
- Islam NU, Jalil K, Shahid M, Muhammad N, Rauf A. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab. J. Chem. 2015;12(8):2310-2319.
- Raffaele C, Luca ID, Luise AD, Petillo O, Calarco A, Peluso G. New therapeutic potential of nanosized phytomedicine. J Nanosci Nanotechnol. 2016;16(8):8176-8187.
Crossref - Chakraborty K, Shivakumar A, Ramachandran S. Nano-technology in herbal medicines: A review. International Journal of Herbal Medicine. 2016;4(3):21-27.
Crossref - Verma HN, Singh P, Chavan RM. Gold nanoparticle: synthesis and characterization. Veterinary World. 2014;7(2):72-77.
Crossref - Yazid H, Adnan R, Hamid SA, et al. Synthesis and characterization of gold nanoparticles supported on zinc oxide via the deposition-precipitation method. Turk J Chem. 2010;34:639-650.
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293-346.
Crossref - Chidambaram J, Ramkumar R, Rahuman AA, Perumal P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Industrial Crops and Products. 2013;45:423-429.
Crossref - Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnology Progress. 2006;22(2):577-583.
Crossref - Song JY, Hyeon KJ, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extract. Process Biochemistry. 2009;44(10):1133-1138.
Crossref - Kaur GJ, Arora DS. In vitro antibacterial activity of three plants belonging to the family Umbelliferae. Inter J Antimicrob Agents. 2008;31(4):393-395.
Crossref - Vitali LA, Beghelli D, Nya PCB, et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab J Chem. 2016;9(6):775-786.
Crossref - Choudhury S, Ahmed R, Kanjilal PB, LeclecqPA. Composition of the seed oil of Trachyspermum ammi (L.) Sprague from Northeast India. J Essent Oil Res. 1998;10(5):588-590.
Crossref - Zarshenas MM, Moein M, Samani SM, Petramfar P. An overview on ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. Journal of Natural Remedies. 2014;14(1):98-105.
- Haiss W, Thanh NTK, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal Chem. 2007;79(11):4215-4221.
Crossref - Rauf A, Qaisar M, Uddin G, Akhtar S, Naveed M. Preliminary Phytochemical Screening and Antioxidant Profile of Euphorbia prostrate. Middle-East Journal of Medicinal Plants Research.2012;1(1):09-13.
Crossref - Uddin G, Rauf A, Siddiqui BS, Muhammad N, Khan A, Shah SU. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyrous lotus L. Phytomedicine. 2014;21(7):954-959.
Crossref - Lu F, Gao Y, Huang J, Huang J, Sun D, Li Q. Roles of biomolecules in the biosynthesis of silver nanoparticles: Case of Gardenia jasminoides Extract. Chin J Chem Eng. 2014;22(6):706-712.
Crossref - Ibrahim HMM. Green synthesis and characterization of silver nanoparticles using Banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci. 2015;8(3):265-275.
Crossref - Mistry B. A Handbook of Spectroscopic Data Chemistry: UV, IR, PMR, CNMR and Mass Spectroscopy, Oxford Book Company, Gujrat. 2009.
- Alfredo VNR, Victor SM, Marco CLA, Rosa GEM, Miguel, CLA, Jesus AAA. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett. 2008;62(17-18):3103-3105.
Crossref - Khan H, Saeed M, Muhammad N, Rauf A, Khan AZ, Ullah R. Antioxidant profile of constituents isolated from Polygonatum verticillatum rhizomes. Toxicol Ind Health. 2016;32(1):138-142.
Crossref - Rehman S, Khan H. Advances in antioxidant potential of natural alkaloids. Current Bioactive Compounds. 2017;13(2):101-108.
Crossref - Uddin G, Rauf A, Siddiqui BS, Khan H, Barkatullah, Ullah R. Antinociceptive, antioxidant and phytochemical studies of Pakistani medicinal plants. Pak J Pharm Sci. 2016;29(3):929-933.
- Muhammad N, Saeed M, Khan H, Haq I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J Nat Med. 2013;67(1):1-8.
Crossref - Asif M, Sultana S, Akhtar N. A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of Trachyspermum ammi Linn. Asian Pac J Trop Biomed. 2014;4(Suppl 2):545-553.
Crossref - Kudavidanage EP, Dissanayake DMI, Keerthirathna WLRK, Nishshanka NLW, Peiris LDC. Commercial formulation of chlorpyrifos alters neurological behaviors and fertility. Biology (Basel). 2020;9(3):49.
Crossref - Garcia DA, Bujons J, Vale C, Sunol C. Allosteric positive interaction of thymol with the GABAA receptor in primary cultures of mouse cortical neurons. Neuropharmacol. 2006;50(1):25-35.
Crossref - Bentley G, Newton S, Starr J. Studies on nociceptive action of α-agonist drugs and their interactions with opioids mechanisms. Br J Pharmacol. 1983;79(1):125-134.
Crossref - Duarte ID, Nakamura M, Ferreira S. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res. 1988;21(2):341-343.
- Khan H, Saeed M, Gilani AUH, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatum verticillatumrhizomes in pain models. J Ethnopharmacol. 2010;127(2):521-527.
Crossref - Ahsan SK, Shah AH, Tanira MO, Ahmad MS, Tariq M, Ageel A M. Studies on some herbal drugs used against kidney stones in Saudi folk medicine. Fitoterapia. 1990;61(5):435-438.
- Srivastava KC. Extract of a spice-omum (Trachyspermum ammi)- shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids. 1988;33(1):1-6.
Crossref - Ratnasooriya WD, Peiris LDC, Jayatunga YNA. Analgesic and sedative action of monocrotophos following oral administration in rats. Medical Science Res. 1995;23(6):403-406.
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.