Abstract
Keywords
Antimicrobial activity Anticancer activity Oxidative stress ent-kaurene Quercetin
Introduction
Globally, microorganisms such as viruses, bacteria, and fungi pose challenges to public health especially in the advent of drug-resistant strains. In recent years, microbial resistance to drugs has attracted considerable attention from researchers in different fields of research including pharmaceutical, food safety and climate change industries, to mention a few. In food industries, E. coli, Listeria, Campylobacter, Staphylococcus and Salmonella species, and yeast have been reported as the predominant causative agents of foodborne diseases (1). Staphylococcus, E. coli, Klebsiella pneumoniae, Acinetobacter species, and Candida species have been implicated to contribute to most common healthcare-associated infections (2, 3). However, because of the increasing pervasiveness of drug-resistant bacterial strains, there is a demand to develop alternative antimicrobial agents to control infections and the spread of diseases. Other degenerative diseases have also increased in recent years and these include cancer, diabetes, atherosclerosis, rheumatoid arthritis, Parkinson’s disease (PD), and Alzheimer’s disease to mention a few.
Cancer is one of the most deadly diseases characterized by cells multiplying continuously spreading malignant cells forming tumors with a possibility of becoming metastatic. During infection, phagocytic cells play an important role in defending the immune system from inflammation and the spread of infections. Through their pattern recognition receptors, phagocytic cells respond to infection, injury or inflammation by secreting various proinflammatory mediators, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), prostaglandin (PG) E2 as well as the free radicals, nitric oxide (NO) and reactive oxygen/nitrogen species (ROS/RNS) (4). The presence of ROS has been linked to many biological processes in normal and cancer cells and these processes include cell signaling (5). They have been said to be two-faced in their cellular functions with many studies highlighting the functions of ROS as tumor-promoting or tumor-suppressing mediators (6, 7) with high amounts of ROS contributing to malignant diseases (8). Maintenance of intracellular balance is necessary to prevent the accumulation of ROS which leads to disease progression. This intracellular defense involves both enzymatic and non-enzymatic antioxidants. Many studies have shown that antioxidants are beneficial for both the prevention and treatment of cancer because they can quench ROS (8). However, these compounds (at certain concentrations or under certain circumstances) may act as prooxidants (9, 10). Despite the d evelopment of treatments (chemotherapy, radiation hormonal therapy) for cancer, it has been reported that patients on chemotherapy experience some adverse effects which may further mutilate their health. In continuation of our studies on S. nigrescens (formerly Acacia nigrescens) (11-13), the biological activities of six S. nigrescens phytocompounds for their antimicrobial, anticancer activity on human breast cancer cell line (MDA-MB-231) and normal murine macrophage cell line (RAW264.7) are reported, and further assessed their suppression of LPS induced oxidative stress.
Experimental
Chemicals, reagents and cells
Solvents (analytical grade) and other chemicals used for the chemistry work were supplied by either Merck (Darmstadt, Germany) or Sigma (St. Louis, MO, USA) chemical companies. Murine macrophage cells (RAW264.7) and breast adenocarcinoma cells (MDA-MB-231) were purchased from Cellonex Separation Scientific SA (Pty) Ltd. (Roodepoort, Johannesburg, South Africa). The Dulbecco’s modified eagle’s medium, fetal bovine serum, and penicillin-streptomycin were bought from Celtic Molecular Diagnostics SA (Pty) Ltd. (Cape Town, South Africa). Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Nutrient agar, Nutrient broth, Sabouraud Dextrose Agar (SDA), Sabouraud dextrose broth (SDB), Ciprofloxacin, Amphotericin-B, Resazurin sodium salt, Doxorubicin, Lipopolysaccharide (LPS) and 2',7'-dichlorodihydrofluorescein diacetate, were purchased from Sigma-Aldrich®, (St. Louis, MO, USA) and Merck (Darmstadt, Germany).
Microbial strains and Microorganisms maintenance
Three microorganisms used in this study, two bacterial strains (Escherichia coli ATCC 25922 - Gram-negative and Staphylococcus aureus ATCC 25923 - Gram-positive) and a fungus (Candida albicans ATCC 10231) were procured from Anatech Instruments (Pty) Ltd, Randburg, Gauteng, South Africa (Anatech, Kwik-stick®). Escherichia coli and S. aureus were cultured in nutrient agar while the fungus (C. albicans ATCC 10231) was cultured on sabouraud dextrose agar (SDA) and incubated for 24 h under aerobic conditions at 37 °C. Pure colonies of microorganisms from the agar were transferred onto nutrient broth and sabouraud dextrose broth (SDB) to obtain an overnight culture.
Plant material
As earlier reported (11), leaves, stem bark, and root of S. nigrescens were collected from the University of KwaZulu-Natal (Westville Campus) in January 2015. Authentication of the plant’s identity was done by Dr. Syd Ramdhani at the School of Life Sciences, University of KwaZulu-Natal. The plant’s voucher specimen with the number “Bodede 02” was deposited at the University’s Ward Herbarium.
Compounds isolation
The procedure for the isolation of compounds 1 – 6 was previously described (11). Briefly, non-polar (combined n-hexane and dichloromethane (DCM)) extract of S. nigrescens stem bark was subjected to column chromatography (CC) using gradient elution (n-hexane:ethyl acetate (EtOAc)) which ran from 10:0 to 0:10. One hundred aliquots of 100 mL each were obtained and combined into 6 fractions based on thin layer chromatography (TLC) analysis. The third and fourth fractions yielded 1 and 2, respectively. Compound 3 was obtained from DCM extract of the root, following a similar chromatographic procedure described for 1 and 2, above. A similar procedure was carried out on the ethyl acetate extract of the stem bark, which gave rise to 55 aliquots from which aliquots 35-43 were further purified on preparative TLC to give compound 4. Compound 5 was obtained by slight modification of the chromatographic procedure described above. The methanol (MeOH) extract of the leaves was partitioned between DCM and EtOAc. The resulting EtOAc fraction was subjected to CC using n-hexane:EtOAc and EtOAc:MeOH solvents systems, starting from 100% n-hexane and finishing with 9:1 of EtOAc:MeOH. The fifteenth aliquot amongst the thirty aliquots collected yielded 5. The EtOAc extract of the root was chromatographed using a similar approach as described above to obtain compound 5. Thirty aliquots were collected and aliquot 20 yielded compound 6.
Sample preparation
Compounds 1 – 6 were weighed and dissolved in 5% Dimethyl sulfoxide (DMSO) to a final working stock solution of 4 mg/mL. Standard antibiotics Ciprofloxacin and Amphotericin-B were also dissolved in 5% DMSO. The samples were kept at 20 °C in the dark until used.
Antimicrobial susceptibility test
Antimicrobial Assay
Antimicrobial activity was done using the microplate serial dilution method by Eloff, (14). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of the compound capable of inhibiting microbial growth. The microorganisms were adjusted according to 0.5 McFarland standard solution to a final concentration of 1.5 × 104 CFU/mL in sterile 0.85% sodium chloride (NaCl) solution. Compounds were tested at the concentration range of 7.8-1000 µg/mL in 96 well plates and incubated with microorganisms for 24 h at 37 °C. Ciprofloxacin and Amphotericin-B were used as positive controls for bacterial and fungal assays, respectively. In addition, sterile distilled 5% DMSO was used as a negative control. A staining reagent, 50 µL Resazurin sodium salt (0.2 mg/mL) was used as an indicator of microbial growth and the MIC was visually determined (15).
Cytotoxicity assay
Cytotoxicity and antiproliferative effects of compounds were evaluated against Murine macrophage cells (RAW264.7) and breast adenocarcinoma cancer cells (MDA-MB-231) using the spectrophotometric, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (16). Cells (5 × 104 cell/well) were seeded in Dulbecco’s modified eagle’s medium (DMEM; Gibco) in a 96 well plate for 24 h incubation at 37 °C in 5% CO2 and treated with compounds (2.5 - 200 µg/mL) and positive control, Doxorubicin (2.5 - 200 µM). Untreated cells and 5% DMSO treated cells were assayed as negative and solvent control, respectively. After 24 h incubation, 20 µL MTT dye (5 mg/mL) was pipetted in all wells including wells with untreated cells, and incubated for 4 h. The medium was aspirated and 100 µL of DMSO was added to all the wells to dissolve the formazan crystals and the optical density (OD) was measured after an hour at 570 nm using a microplate reader.
Effects of compounds on intracellular ROS production in RAW264.7
Lipopolysaccharide (LPS)-induced reactive oxygen species (ROS) production was probed using an oxidative cell-permeant fluorescent dye 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA) (17). RAW264.7 cells (5 × 104 cells/well) were seeded overnight into a 96 well plate and treated with compounds (10 µg/mL) at concentrations ≤ IC50 values and LPS (10 µg/mL) for 24 h at 37 °C in 5% CO2 rich incubator, then 10 μM H2DCF-DA dye (100 µL) was added for an hour. The fluorescence intensity which correlates to ROS production was measured on a microplate reader at 485 and 535 nm excitation and emission, respectively. Data obtained were subjected to analysis using GraphPad Prism (version 8.2).
Statistical analysis
Experiments were performed in triplicate and analyzed using a microplate reader (Varioskan Flash, Thermo Fisher Scientific, Vantaa, Finland). Results were presented as means ± standard deviation (SD). One-way ANOVA and Duncan multiplication range test was used to differentiate between means. Graphs and IC50 values were calculated using the GraphPad prism software (version 8.2). **p < 0.01; ***p < 0.001. was considered significant.
Results and Discussion
Antimicrobial susceptibility test
The antimicrobial activity of compounds 1-6 (Figure 1) obtained from S. nigrescens tested over the concentration range 7.81 - 1000 µg/mL exhibited varying minimum inhibitory activity as shown in Table 1. Compounds showed MIC values ranging from 31.25 - 1000 µg/mL and for this study, MIC values that are not greater than 10-fold the antibiotic control were not considered significant. Compounds 5 and 6 showed good activity followed by compounds 4 and 3. However, compound 3 had an insignificant MIC of 250 µg/mL against E. coli. Compounds 1 and 2 had MIC values ≥ 250 µg/mL across the three strains.
Both lupane-type triterpenes (1 and 2) had the same MIC values across the strains indicating that the difference in position 30 substitution (-CHO and -CH2OH) did not impact the antibacterial and antifungal activity of the compounds. Better activities were observed for the ent-kaurene (3) in S. aureus (MIC: 125 µg/mL) and C. albicans (MIC: 62.5 µg/mL). This may be due to the presence of a bicyclic system in the kaurenoid skeleton and lower molecular weight in comparison to triterpenes 1 and 2. Earlier, it was reported that the flavonols 4, 5, and 6 had promising antibacterial and anti-quorum sensing activities which in this study was extended to the bacterial strain, S. aureus ATCC 25923, and the fungal strain, C. albicans ATCC 10231. Thus, the antimicrobial spectrum of 4, 5, and 6 may include antifungals in addition to their bacterial quorum sensing inhibition potential.
Overall, all tested samples showed higher MIC against E. coli compared to S. aureus and C. albicans, which agrees with the notion that gram-negative bacteria like E. coli are more resistant than their positive counterparts.
Cytotoxicity
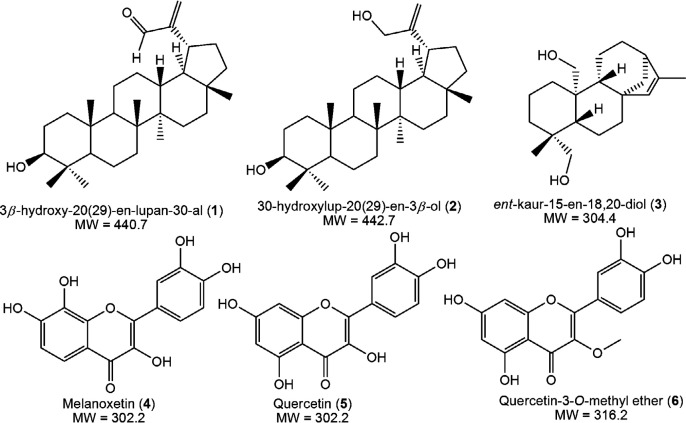
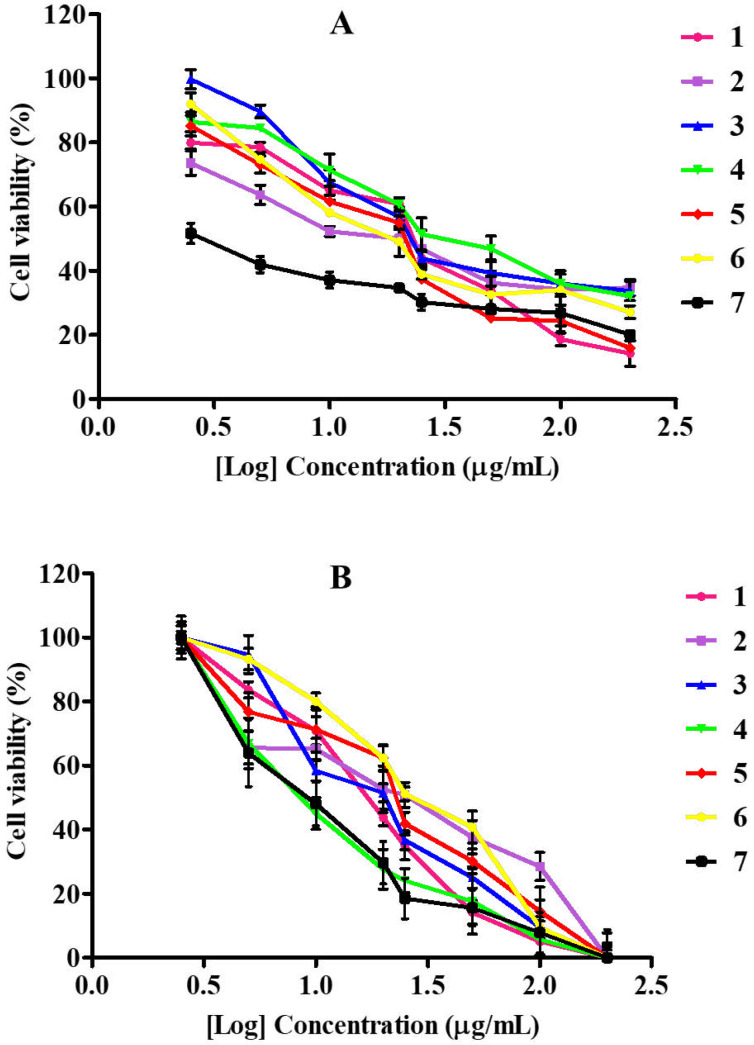
Cytotoxicity evaluated with the MTT assay on cancerous (MDA-MB-231) and non-cancerous (RAW264.7) cell lines revealed a dose-dependent effect when treated with pure compounds isolated from S. nigrescens with a concentration range of 200 - 2.5 µg/mL for 24 h. Tested concentrations showed no statistical differences between compounds treated cells and positive control, doxorubicin (Figure 2A). On the breast cancer cells, compounds 1, 4 and 5 had IC50 values of 26.65, 22.17 and 20.61 µg/mL, respectively, while those of 2 (11.86 µg/mL), 3 (12.62 µg/mL) and 6 (14.03 µg/mL) were found to be comparable to the IC50 value of doxorubicin (9.35 µg/mL) (Table 2). For anticancer activity, IC50 values below 15 µg/mL are considered significant as they had 50% mortality of the cell population at a lower concentration (Table 2). An earlier cytotoxicity study on A. mellifera derived lupane-type triterpenes revealed that at least, presence of one hydroxyl group is required for expressing activity (18), which may be responsible for the observed activities of 1 and 2 in the present study. However, the α,β-unsaturated alcohol system on 2 had a better influence on cytotoxicity compared to α,β-unsaturated aldehyde system on 1. The new ent-kaurene (3) also showed significant activity which may be due to the presence of two hydroxyl groups as observed for 2. Sarwar et al. (2020) recently unveiled the molecular targets and mechanistic pathways of ent-kaurenes with significant anticancer potential (19). Ent-16β-17α-dihydroxykaurane (DHK), with a molecular weight of 306.4 had the most structural similarity to (3). Amongst the flavonols, quercetin-3-O-methyl ether (6) had the highest cytotoxic effect on MDA-MB-231 which is consistent with a recent study that confirmed its potency against the triple-negative breast cancer through extensive cytotoxicity, apoptosis and mechanistic studies (20). Although quercetin (5) was initially identified as a potential anticancer candidate (21, 22), further research brought to light its limitations (such as poor water solubility, bioavailability, easy oxidation, and toxicity to normal cells) (23) and its analogs and nano-hybrids subsequently developed, presented improved activities and few side effects (23, 24). It has been established that quercetin reaches the bloodstream in the form of different bio-transformed species including the methylated analogs (25). Thus, the better result of 6 compared to 5 makes the 3-O methyl derivative preferred over quercetin being the expected bioavailable species under in-vivo conditions.
To evaluate the compounds’ selective cytotoxicity, the compounds were tested on RAW264.7, which are normal immune cells. All compounds affected cell viability in a dose-dependent manner (Figure 2B) with their corresponding IC50 values presented in Table 2. All compounds had low IC50 values, with 2, 5 and 6, having 22.81, 22.61, 28.73 µg/mL, respectively. The IC50 values of compounds 1, 3 and 4 were 16.89, 18.50, 15.80 µg/mL, respectively, while the positive control was more toxic with an IC50 value of 10.61 µg/mL. Upon comparison of the compounds’ toxicity on RAW264.7 to the cancer cells, 1 and 4 were more toxic to the normal cells than the cancer cells. The triterpene 2 showed better selectivity (SI, 1.92) than the diterpene 3 (SI, 1.46), whereas the well-known quercetin (5) had low selectivity with SI (1.09) comparable to the control, doxorubicin (1.13). These findings suggest that the α,β-unsaturated alcohol moiety of 2 is preferable to α,β-unsaturated carbonyl moiety of 1 for selective cytotoxicity. The positions of hydroxy substitutions on the flavonol skeleton probably play a significant role in the selectivity observed across 4, 5 and 6. Compound 6, the 3-O methyl ether derivative of quercetin was most selective to the carcinoma cells with the highest selectivity index of 2.04. The toxicity of bioactive compounds to non-cancerous cells remains a great concern in cancer chemotherapy. More work is still required on the modification of plant-derived therapeutic agents for better cytotoxic selectivity.
Intracellular ROS production in RAW264.7
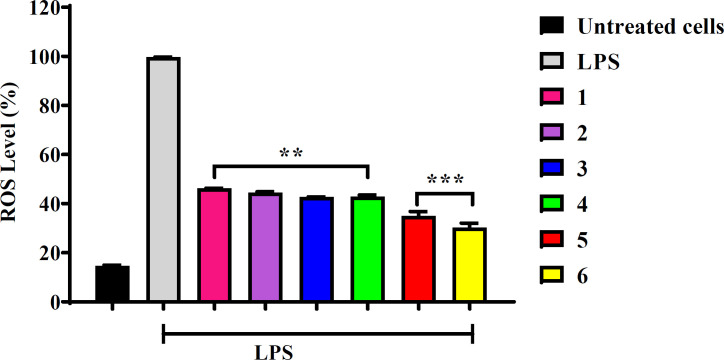
Inflammation is a physiological response stimulated by the organism as a way of overcoming pathogenic events employed as a defense mechanism. However, an unregulated inflammatory response can lead to several diseases, including cancer. ROS-inducing approaches rely on the fact that increasing the ROS level over the cytotoxic threshold can result in cellular impairment, damaging DNA, RNA, proteins and lipids in normal cells, even though macrophage cells cannot phagocytize cancerous cells, but release a reasonable amount of pro-inflammatory cytokines that quench cancer cells yet are cytoprotective to normal cells (6). In this regard, it was considered that the compounds tested would be able to reduce the production of LPS-induced ROS in normal macrophages. Determination of ROS production in RAW264.7 cells was conducted using the non-toxic IC50 value of 10 µg/mL for all compounds (Table 2). Cells were pre-treated with compounds for 20 h and LPS was added in all treated cells except the untreated control cells. Thereafter H2DCFDA was introduced to the cells. As observed in Figure 3, all tested compounds significantly decreased the ROS production compared to the LPS-treated cells. Due to high metabolic activity, cancer cells produce high levels of ROS to evade immune anticancer response. However, the continuous elevation of ROS from cancer cells infiltrates immune cells by inducing oxidative stress. Therefore, the basis of conducting this investigation on normal (Raw264.7) cells was to assess the protective power of the compounds against ROS toxicity, which leads to oxidative stress and cells death. However, the possible mechanism of ROS reduction effect in cells treated with compounds might be associated with the direct free radical scavenging activity or indirect protection from oxidative stress.
Flavonoids and terpenoids are known for their antioxidant and anti-inflammatory properties (26-29). Quercetin and related flavonols have shown potentials as anti-inflammatory agents. Kim et al. (2004) reported that quercetin down-regulated the release of LPS-induced pro-inflammatory mediators including IL-1, IL-6, TNF-α in RAW 264.7 cells (30). Recently, it was found that quercetin significantly reduced ROS intracellularly, thereby protecting L02 cells from D-galactosamine (D-GaLN)-induced damage (31). The present study demonstrated that quercetin (5) and its 3-O-methyl ether (6) significantly reduced ROS production to levels below 50% (Figure 3), which agrees with previous findings. However, contrasting reports exist where quercetin and similar flavonols upregulated the LPS-induced release of proinflammatory mediator IL-6 and IL-8 as opposed to other flavonoid groups like flavones, isoflavones, flavanes and chalcones which reduced the same interleukins (IL-6 and IL-8) (32). Melanoxetin (4) and the terpenoids (1–3) tested in this study exhibited approximately 40% reduction of ROS. Pharmacological studies of the phenolic compounds in Acacia confusa revealed that 4 is a strong inhibitor of LPS-induced ROS and RNS with IC50 values of 12.5 μM and 6.9 μM, respectively (33, 34).
Mediation of oxidative stress by regulating pro-inflammatory cytokines initiates the lipid peroxidation process and may lead to the damage of bacterial/cell membrane, thereby proposing a possible mechanism of antimicrobial and anticancer activity. To support this view, studies conducted on the antimicrobial mechanisms of action of flavonoids proposed mechanisms such as inhibition of nucleic acid synthesis, interruption of the cytoplasmic membrane functions and disruption of energy metabolism (35, 36). However, the mechanisms are not limited to flavonoids but include terpenoids (37). Resveratrol and quercetin reduced the nitric oxide (NO) production in Salmonella typhimurium infected human myeloid leukemia cell line (U937 cells), as a result, cell viability and proliferation in infected cells were inhibited. Moreover, the compounds showed protective effects of the host cells from the toxic effects of bacterial infection and decreased programmed cell death (38). Furthermore, quercetin and quercetin-3-O-rhamnoglucoside exerted their antimicrobial activity by reducing the bilayer thickness of microorganisms (39). It is also likely that the in vitro microenvironment of quercetin and its derivatives (as seen in the present study), creates a redox condition that permits a protective ROS inhibition or pro-oxidant antimicrobial function. Apigenin-8-C-glucoside was shown to exhibit antimicrobial activity against S. aureus and its mechanism was suggested as reducing the hydrophobicity of cell surface and membrane permeability at an MIC = 126 µg/mL (40).
Conclusion
Six S. nigrescens compounds exhibited strong biological activities when evaluated for their antimicrobial, cytotoxicity, and reactive oxygen species inhibitory capacity. Generally, compounds 5 and 6 showed strong antimicrobial activity and good activity in reducing cancerous viable cells. All compounds suppressed the LPS-induced ROS levels. Our findings further justify the ethnomedicinal use of S. nigrescens whose phytoconstituents possess remarkable therapeutic potentials. The antimicrobial activity of the compounds could be extrapolated to the plant’s potential for treating wounds and skin diseases. The low cytotoxic selectivity of the compounds suggests that care must be taken in the therapeutic usage of single-agent plant-derived metabolites. Prospective studies should be done to understand the mechanism of action especially investigating the regulation of mitogen-activated protein (MAP) kinases and nuclear factor (NF)-κB cascades and proinflammatory mediators including TNF-α, interleukins (IL), and MIP-2 secretion by compounds tested in this study.
Author’s contributions
Conceptualisation: Olusola Bodede
Investigation and data curation: Garland K. More and Olusola Bodede
Project administration and supervision: Gerhard Prinsloo
Writing, review, editing and approval of final manuscript: All authors
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
References
-
1.
Yeni F, Yavaş S, Alpas H, Soyer Y. Most common foodborne pathogens and mycotoxins on fresh produce: a review of recent outbreaks. Crit. Rev. Food Sci. Nutr. 2016;56:1532-44. [PubMed ID: 26583913].
-
2.
Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010;362:1804-13. [PubMed ID: 20463340].
-
3.
Agaba P, Tumukunde J, Tindimwebwa J, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res. Notes. 2017;10:349. [PubMed ID: 28754148].
-
4.
Du C, Bhatia M, Tang SC, Zhang M, Steiner T. Mediators of inflammation: Inflammation in cancer, chronic diseases, and wound healing. Mediators Inflamm. 2015.
-
5.
Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:2407.
-
6.
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev. 2019.
-
7.
Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol. Ther. 2008;7:1875-84. [PubMed ID: 18981733].
-
8.
Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031-44. [PubMed ID: 12049739].
-
9.
Rietjens IM, Boersma MG, de Haan L, Spenkelink B, Awad HM, Cnubben NH, van Zanden JJ, van der Woude H, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002;11:321-33. [PubMed ID: 21782615].
-
10.
Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513-23. [PubMed ID: 21277359].
-
11.
Bodede O, Shaik S, Chenia H, Singh P, Moodley R. Quorum sensing inhibitory potential and in silico molecular docking of flavonoids and novel terpenoids from Senegalia nigrescens. J. Ethnopharmacol. 2018;216:134-46. [PubMed ID: 29408657].
-
12.
Shaik S, Bodede O, Moodley R. Senegalia nigrescens: clonal propagation and bioactive compounds from calli. S. Afr. J. Bot. 2018;115:329.
-
13.
Bodede O, Shaik S, Moodley R. Evaluation of bioactive flavonols and ent-kaurenes in the 2, 4-dichlorophenoxyacetic acid-induced calli of Senegalia nigrescens using FTIR and GC–MS. J. Plant Biochem. Biot. 2021:1-8.
-
14.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711-3. [PubMed ID: 9933989].
-
15.
-
16.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63. [PubMed ID: 6606682].
-
17.
Chen SK, Hsu CH, Tsai ML, Chen RH, Drummen GP. Inhibition of oxidative stress by low-molecular-weight polysaccharides with various functional groups in skin fibroblasts. Int. J. Mol. Sci. 2013;14:19399-415. [PubMed ID: 24071940].
-
18.
Mutai C, Abatis D, Vagias C, Moreau D, Roussakis C, Roussis V. Cytotoxic lupane-type triterpenoids from Acacia mellifera. Phytochemistry. 2004;65:1159-64. [PubMed ID: 15110698].
-
19.
Sarwar M, Xia YX, Liang ZM, Tsang SW, Zhang HJ. Mechanistic pathways and molecular targets of plant-derived anticancer ent-kaurane diterpenes. Biomolecules. 2020;10:144.
-
20.
Cao L, Yang Y, Ye Z, Lin B, Zeng J, Li C, Liang T, Zhou K, Li J. Quercetin3methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2018;42:1625-36. [PubMed ID: 29956731].
-
21.
Zimmerman NP, Peiffer D, Stoner GD. Cancer prevention by antioxidant compounds from Berries. In: Kong ANT, editor. Inflammation, oxidative stress, and cancer: dietary approaches for cancer prevention. CRC Press, Boca Raton, FL, USA; 2013. p. 419-32.
-
22.
Rauf A, Imran M, Khan IA, ur‐Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: a comprehensive review. Phytother. Res. 2018;32:2109-30. [PubMed ID: 30039547].
-
23.
Zhou Y, Chen D, Xue G, Yu S, Yuan C, Huang M, Jiang L. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 2020;10:34517-26.
-
24.
Massi A, Bortolini O, Ragno D, Bernardi T, Sacchetti G, Tacchini M, De Risi C. Research progress in the modification of quercetin leading to anticancer agents. Molecules. 2017;22:1270.
-
25.
Almeida AF, Borge GIA, Piskula M, Tudose A, Tudoreanu L, Valentová K, Williamson G, Santos CN. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf. 2018;17:714-31. [PubMed ID: 33350133].
-
26.
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016:7432797.
-
27.
Prakash V. Terpenoids as source of anti-inflammatory compounds. Asian J. Pharm. Clin. Res. 2017;10:68-76.
-
28.
Choy KW, Murugan D, Leong XF, Abas R, Alias A, Mustafa MR. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: a mini review. Front. Pharmacol. 2019;10:1295. [PubMed ID: 31749703].
-
29.
Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020;15:1-13.
-
30.
Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004;96:229-45. [PubMed ID: 15539763].
-
31.
Fang P, Liang J, Jiang X, Fang X, Wu M, Wei X, Yang W, Hou W, Zhang Q. Quercetin attenuates D-GaLN-induced L02 cell damage by suppressing oxidative stress and mitochondrial apoptosis via inhibition of HMGB1. Front. Pharmacol. 2020;11:608. [PubMed ID: 32431618].
-
32.
Zhang P, Mak JC, Man RY, Leung SW. Flavonoids reduces lipopolysaccharide-induced release of inflammatory mediators in human bronchial epithelial cells: structure-activity relationship. Eur. J. Pharmacol. 2019;865:172731. [PubMed ID: 31610186].
-
33.
Wu JH, Tung YT, Chien SC, Wang SY, Kuo YH, Shyur LF, Chang ST. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J. Agric. Food Chem. 2008;56:1567-73. [PubMed ID: 18254591].
-
34.
Lin HY, Chang TC, Chang ST. A review of antioxidant and pharmacological properties of phenolic compounds in Acacia confusa. J. Tradit. Complement. Med. 2018;8:443-50. [PubMed ID: 30302324].
-
35.
Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr. Med. Chem. 2015;22:132-49. [PubMed ID: 25245513].
-
36.
Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019;18:241-72.
-
37.
Oz M, Lozon Y, Sultan A, Yang KHS, Galadari S. Effects of monoterpenes on ion channels of excitable cells. Pharmacol. Ther. 2015;152:83-97. [PubMed ID: 25956464].
-
38.
Paolillo R, Carratelli CR, Rizzo A. Effect of resveratrol and quercetin in experimental infection by Salmonella enterica serovar Typhimurium. Int. Immunopharmacol. 2011;11:149-56. [PubMed ID: 21093605].
-
39.
Sanver D, Murray BS, Sadeghpour A, Rappolt M, Nelson AL. Experimental modeling of flavonoid–biomembrane interactions. Langmuir. 2016;32:13234-43. [PubMed ID: 27951697].
-
40.
Das MC, Das A, Samaddar S, Daware AV, Ghosh C, Acharjee S, Sandhu P, Jawed JJ, De UC, Majumdar S. Vitexin alters Staphylococcus aureus surface hydrophobicity to interfere with biofilm formation. BioRxiv. 2018:301473.