Abstract
Keywords
Ferulago Essential oil Pharmacological activity Herbal medicine Phytotherapy
Introduction
Herbal used in traditional and modern medicine presents a valuable source of secondary metabolites and different pharmacological and biological activities. Hence, they could be utilized as a lead compound to produce new drugs and treat numerous diseases (1). The Apiaceae or Umbelliferae, commonly known as the parsley, carrot, or celery family, is one of the biggest plant families in the world usually characterized by aromatic plants with hollow stems (2). Several plants belonging to this family are well-known as vegetables, culinary, and medicinal plants, including Apium gravolence, Foeniculum vulgare, Centella asiatica, Pimpinella anisum, Cuminum cyminum, Carum carvi, Ligusticum officinale, Coriandrum sativum, Anethum graveolens , Ammi visnaga, Anthriscus cerefolium, Ferulago angulata, and Ferula assa-foetida (3). Plants of the Apiaceae family are commonly aromatic and pungent owing to the existence of essential oil or oleoresin in their diverse organs (4). Among several Umbelliferae families, the species belonging to the genus Ferulago W. Koch. are broadly employed in traditional medicine. The genus Ferulago W. Koch. (called Chavil or Chavir in Persian, Çakşır or Çağşır in Turkey) appertains to the Umbelliferae family consisting of 49 species which are mostly distributed in Asia, Europe, and Africa (5, 6).The occurrence of several bioactive secondary metabolites, including coumarin, coumarin esters, furanocoumarin, flavonoids, quinones, steroids, essential oils, steroids, and terpenoids are owing to their pharmacological activities (7). Extensive studies have been conducted to evaluate the ethnomedicinal uses of Ferulago species antibacterial, antioxidant, Alzheimer, anti-diabetics, anti-malaria, anti-coagulant, and aphrodisiac effects. The information of this review paper has been collected from journals available in databases including Science Direct, Web of Science, Scopus, PubMed, EBSCO, Google Scholar, and Hindawi up to March 2020. The keywords and search terms contained “Ferulago”, “Ferulago spp.” “essential oil of Ferulago”, “phytochemistry and Ferulago”, “coumarins and Ferulago”, “bioactive compounds and Ferulago”, “pharmacological activity and Ferulago”. Therefore, we conducted the present review article to provide a complete overview of a current state of knowledge on botany, ethnomedicinal uses, phytochemistry, and the most noticeable pharmacological effects of species belonging to the genus Ferulago.
The botany of Ferulago plants
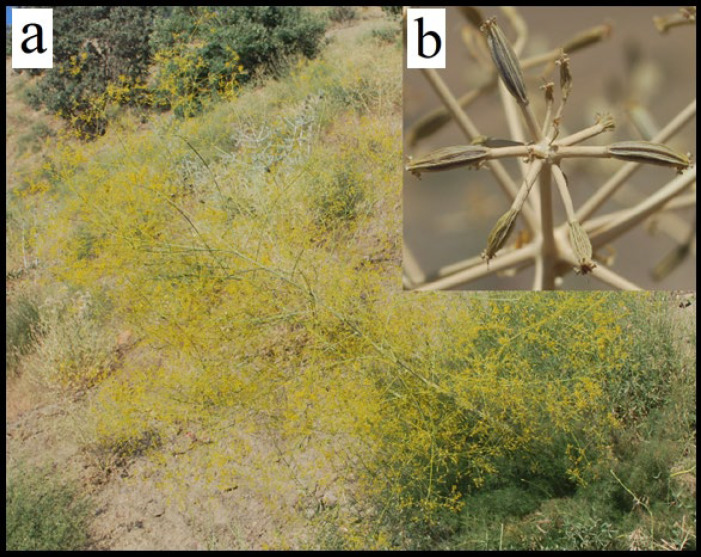
The Ferulago genus appertains to the Umbelliferae family and comprises 49 species which are mainly distributed in Asia, Europe, and Africa (8). The genus Ferulago is distributed in Turkey with 34 species, out of which 18 are endemic (9). The 8 species exist in Iran, out of which three are endemic (10). According to The Plant List website, the genus Ferulago consists of 48 accepted species names and 13 unassessed species(11). Table 1 depicts the name of accepted species and their synonyms. Ferulago genus is 60-150 cm tall; annual or perennial plants grow at altitudes of 1900- 3200m above sea level with yellow fruits. They are characterized by persistent bracts and bracteoles and small flowers which are widely distributed in Turkey, Iraq, and west of Iran (from the flora of Iran, particularly in Kermanshah, Ilam, Lorestan, and Kurdistan) (8, 12, 13). Pictures of Ferulago bernardii and fruits of ferulago stellata are shown in Fig.1. The high distribution of Ferulago spp. in these areas suggests that the core center of the biodiversity of the genus Ferulago is in Anatolia. Based on taxonomy the species of this genus resemble Prangos and Ferula species. They are widely used in the Anatolia region for several purposes (7, 14). The most studied species is F. angulata which is divided based on ovaries, the flowering of in fluorescence, and fibers (rather absence or presence of trichomes) into two subspecies: F. angulate subsp. angulata and F. angulate subsp. Carduchorum by Chamberlain in 1987(15).
Traditional use of Ferulago spp.
Ferulago like Ferula and Prangos species have been traditionally utilized back in history in folk medicine for the medicament of hemorrhoids, and intestinal worms, peptic, sedative, carminative, digestive, aphrodisiac, snakebite, wound skin infections, headache, diseases of the gastrointestinal tract and spleen (5, 16). Due to the aromatic property of Ferulago, some of them have been applied in Iranian tradition as spices in different foods, such as dairy and meat products, for increasing the flavor and as an aroma. They have also been employed as natural preservatives for enhancing food expiration dates (8, 16 and 17). Bakhtiari nomads use F. angulata to make some foods and Nomads of Fars province utilize this plant to flavor yogurt. The F. angulata is also used as a sedative, food-digestive, tonic, antibacterial, and antiparasitic(18). In Turkish traditional medicine, the aerial parts of a few members of this genus are used as an immunostimulant, tonic, sedative, digestive, anti-bronchitis, flavor, vermicidal, anthelmintic, and anti-peptic (19, 20). Likewise, the root parts are used for the treatment of dermatological disorders and cancers and as an aphrodisiac and are preferred as fodder to improve animal productivity (7). The seeds are applied for eye pains in the form of inhalation (8). It has been reported that dried or fresh leaves of Ferulago are used as foot deodorant by indigenous people of the north of Iraq (21).
Phytochemistry of essential oils from the genus Ferulago species
Essential oils or volatile oils are the phytochemical complexes of different aromatic components, mainly monoterpenoids and sesquiterpenoids, which are obtained from plant materials, for instance, leaves, fruits, seeds buds, flowers, roots, and bark. They are characterized by having a strong odor generally lower density than water, being volatile, rarely colored, liquid, lipophilic, and soluble in organic solvents (7). Despite the way that these volatile oils involve around 20–60 constituents, just a few of them exist at high amounts (20–70%) in correlation with different constituents existing in low sums (22). These phytochemicals assume a vital part in the protection of the herbs from herbivores, insects, bacteria, fungi, viruses, and also help to attract pollinators (23). These essential oils could be extracted with conventional methods (steam distillation, hydrodistillation (HD), organic solvent extraction) and innovative techniques (In situ microwave-generated hydrodistillation, supercritical carbon dioxide, microwave steam diffusion, microwave hydro diffusion and gravity, and microwave steam distillation). Among these methods, the hydro-distillation method is the most prevalent technique for obtaining essential oils (7, 24 and 25). The method of extraction, drying methods, genotypic variation, geographical origin of the plant, stage of the development, and part of the plant used may drastically affect the composition of essential oils of plants (26-28). Based on the literature, researches have revealed that the species of the Ferulago genus are appropriate for the extraction of essential oils and many of them have been evaluated for chemical compositions, including F. macedonica, F. angulata, F. carduchorum, F. phialocarpa, F. contracta, F. macrocarpa, F. blancheana, F. bernardii, F. pachyloba, F. longistylis, F. isaurica, F. syriaca, F. platycarpa, F. thyrsiflora, F. sylvatica, F. nodosa, F. pauciradiata, F. asparagifolia, F. aucheri, F. galbanifera, F. confusa, F. humilis, F. campestris, F. idaea, F. macrosciadia, F. mughlae, F. sandrasica, F. silaifolia, F. trachycarpa, F. thirkeana, F. setifolia, F. subvelutina, F. stellata, F. capillaries F. trifida. Table 2 represents the main compounds of these essential oils, part of the herb that was used, methods of extraction, percentage yield, class of components, and their references.
According to data of the volatile oils extracted from various Ferulago spp., it is clear that every species and each part of the plant have a diversified set of main compounds. Therefore, it is hard to find the similarity among the species of this genus concerning the chemicals. Several compounds, like α-Pinene, p-Cymene 2,3,6-trimethyl benzaldehyde, cis-Chrysanthenyl acetate, α-Phellandrene, Sabinene, (Z)-β-Ocimene, Limonene, Myrcene, Terpinolene, Nonacosane, and δ-Cadinene, have been detected as main components of the volatile oils of many Ferulago species. To date, analysis has shown that the α-Pinene is a major compound of several Ferulago species; hence, this might be regarded as a perpetual constituent for the genus (7). There are certain other notable points to report; primarily, the compound of spathulenol was the major component from aerial parts of only two species namely, F. sylvatica and F. thyrsiflora (45). Secondly, the compound of ferulagone was found as the main compound of only F. thirkeana obtained from its fruits (59). Thirdly, carvacrol methyl ether obtained with MD from fruits of F. macrosciadia and F. idaea was the major component of only these two species (47). Furthermore, Cecchini et al. compared the composition of the volatile oil from roots and fruits of F. campestris from two sites of collection and two periods of time. They found significant differences in the percentages of (2, 30 and 6) ‐trimethyl benzaldehyde (14.8–27.9% in the roots gathered in the summer, 65.2% in roots gathered in the fall) and α-Pinene (58.3-75% in the roots gathered in the summer, 19.3% in the roots gathered collected in the fall) (23).
Phytochemistry of plant extracts from the genus Ferulago species
Phytochemical investigations on Ferulago species have shown the presence of various secondary metabolites including coumarins, coumarin esters, furanocoumarins, aromatic compounds, monoterpenes, sesquiterpenes, flavonoids, quinones, and stilbene. Table 3 depicts the main phytochemicals that have been isolated and characterized from Ferulago species. Up to now, several studies have been done to distinguish active compounds from different parts of the ferulago genus, from which about 73 (three simple coumarins, sixteen furanocoumarins, five dihydro-furanocoumarin, four sesquiterpene coumarin, twelve prenylated coumarins, six pyranocoumarin, nine flavonoids, and eighteen miscellaneous compounds) bioactive compounds were isolated. Based on the literature, coumarins and their derivatives are the most prevalent secondary metabolites on the Ferulago species and might be used as a chemotaxonomic marker in the genus Ferulago (65). The classification of different types of coumarins and various biological applications of each compound was reviewed by Venugopala et. al. (66).
Pharmacological activities
In the last two decades, many ferulago species have been widely studied with advanced scientific methods and reported for several pharmacological properties such as antibacterial activity, antioxidant activity, antidiabetic activity, larvicidal activity, Alzheimer, and anticancer activity. These pharmacological activities of Ferulago are considered to be attributed mainly to its coumarins and furanocoumarins and essential oil (66). Table 4 depicts pharmacological activity and model of study of the genus Ferulago.
Antibacterial activity
The volatile oils of many herbs of genus Ferulago have been the focus on pharmacological activity, particularly from an anti-oxidant, antimicrobial and antifungal point of view (7, 20 and 29). In the literature, antimicrobial and antifungal properties of essential oil were screened versus Gram-positive (Staphylococcus aureus, S. epidermidis, and methicillin-resistant S. aureus), and Gram-negative (Escherichia coli, Salmonella typhimurium, Bacillus cereus, Proteus vulgaris, Enterobacter aerogenes, and Pseudomonas aeruginosa) bacteria, and the yeast (Candida albicans, C. parapsilosis, and C. tropicalis) via broth microdilution assay. Sucu et al., 2019, studied the antimicrobial effects of volatile oil from the roots and aerial parts of F. sandrasica and found that both essential oils were not active against C. tropicalis and C. parapsilosis compared to positive controls (55). The volatile oil of the aerial portions was found to be active against Salmonella typhimurium, Staphylococcus aureus, and Bacillus subtilis, however, inactive against E. coli; the root essential oil was active against B. subtilis and S. typhimurium compared with E. coli, but not active against S. aureus. Recently, Karakaya et al. assessed antimicrobial activity of n-butanol, ethyl acetate, dichloromethane, methanol extracts, and aqueous residue parts of methanol extracts from the aerial parts and roots of four Ferulago species (F. pachyloba, F. bracteata, F. trachycarpa, and F. blancheana) along with 14 isolated compounds via micro broth-dilution methods. Their result demonstrated that the best antimicrobial effect against B. subtilis, E. coli, S. aureus, P. aeruginosa, and C. albicans were obtained with methanol extract of the roots, n-butanol fractions, and methanol extract of the aerial parts from F. blancheana (62.5 µg/mL), dichloromethane fraction of the roots and aerial parts from F. pachyloba (62.5, 31.25 µg/mL), the n-butanol fraction of the aerial parts, dichloromethane fractions of the aerial parts and roots, methanol extracts and ethyl acetate fraction of the roots from F. bracteata (62.5 µg/mL), dichloromethane fraction of roots, and methanol extracts of roots and aerial parts from F. trachycarpa (62.5 µg/mL) and prantschimgin (31.25 µg/mL) (78). According to them, the E. coli was less affected than the other microorganisms; the best activity against C. albicans (MIC = 31.25 μg/mL) was obtained by the CH2Cl2 fraction of aerial portions from F. pachyloba and isolated compound prantschimgin. Furthermore, Pinto et al. evaluated the antifungal effects of an essential oil, and two main compounds of it on germ tube formation, ergosterol biosynthesis, and mitochondrial function. Limonene presented a weaker activity (0.32 to 20 μL/mL) than the essential oil and α-pinene with low and similar to MIC and MFC values against the tested organisms (0.08 to 5.0 μL/mL). The essential oil of F. capillaris suppressed germ tube formation at sub-inhibitory dose on Candida albicans. The mechanism of antifungal activity of F. capillaris indicated no distribution on the ergosterol content and defect of mitochondrial role in a dose-dependent way in essential oil-treated C. albicans (62).
Anti-oxidant effect
In recent decades, extensive studies have been conducted on the evaluation of the antioxidant function of medicinal plants as a source of natural compounds not only to combat several degenerative disorders, including cardiovascular disease and cancer, but also as a substitute compound to artificial additives, like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) in food productions (94). Anti-oxidant constituents diminish the extent of oxidative damage via acting as free radical scavengers (95). There are numerous methods, such as radical scavenging power, reducing power, and inhibition of lipid peroxidation in a β-carotene–linoleate system for assessing the antioxidant activity of plant extracts or volatile oils (96). The DPPH radical-scavenging method is one of the easiest and rapid tests for evaluating the antioxidant activity of natural compounds(16). According to literature, an antioxidant activity study has been performed on F.macrocarpa, F. carduchorum (97), F. bernardii (16), F. sandrasica, F.macedonica, F. trifida (64), F. subvelutina (74), F. cassia (8), F. angulata, and F. campestris (79). The antioxidant activities of four fractions and crude extract of aerial portions of F. carduchorum at 2 vegetative periods (flower and fruit) were assessed using the DPPH method. The best activity belonged to flower crude extract (IC50 = 0.44 mg/mL)(97). Shahbazi and Shavisi investigated the antioxidant activities of nonpolar and polar sub-fractions of methanolic extract, and the volatile oil of the aerial parts of F. bernardii and compared them to BHT via DPPH assay. The antioxidant activity with the mean of IC50 were polar sub-fractions (5.66), non-polar sub-fractions (6.88), and essential oil (14.81), while they displayed lower radical scavenging activity compared with BHT(16). Tavakoli et al. found that due to lack of phenolic compounds in the conformation of the volatile oil of different parts of F. trifida, a feeble free radical scavenging effect was observed (IC50: 95–120 μg mL−1) compare to BHT (IC50: 21.2 ± 2.6 μg mL−1) (64). Moderate antioxidant activities were attained with DPPH test from isolated coumarin of the roots of F. subvelutina compare to BHT (IC50 = 27 µg/mL) > oxypeucedanin hydrate (IC50= 160 µg/mL) >meranzin hydrate (IC50 = 180 µg/mL) >osthole(IC50 = 27 µg/mL) >oxypeucedanin (IC50= 217 µg/mL) >isoimperaturin(IC50 = 245 µg/mL) > xanthotoxin (IC50= 270 µg/mL) (74). In another study, the antioxidant activity of isolated compound, and the extracts and fractions of the aerial parts, fruits, flower, and roots of F. cassia were investigated via TBA assay. The highest antioxidant potential was obtained based on following order: Peucedanol (IC50 = 18.1 µg/mL)>Suberosin (IC50 = 23.5 µg/mL)> roots CH2Cl2fractions (IC50 = 43.1 µg/mL)> fruit CH2Cl2 fractions (IC50 = 54.4 µg/mL) > Grandivitinol (IC50 = 61.1 µg/mL) > Umbelliferone (IC50 = 79.5 µg/mL) (8).
Alzheimer’s disease (AD)
Alzheimer’s is one of the neurological disorders with decreasing acetylcholine followed by the deterioration of short-term memory, which occurs in elderly people mostly based on genetic inheritance. One promising strategy for combating AD is using anticholinesterases or acetylcholinesterase inhibitors (AChEIs) to inhibit the hydrolysis of acetylcholine and raise its level in the synaptic cleft (82). Hajimehdipoor and co-worker studied the acetylcholinesterase inhibitory property of the total extracts and fractions of the aerial portions of F. subvelutina and the whole plant of F. angulata employing the Ellman method. Their result implied that a total extract of both Ferulago genes can inhibit the acetylcholinesterase enzyme with 19.7% and 15.8% respectively for F. subvelutina and F. angulata. The dichloromethane fraction displayed the highest AChEI activity among all the fractions (98). Golfakhrabadi et al. found that all the coumarins obtained from the aerial parts of F. carduchorum have AchE enzyme inhibition, among which xanthotoxin revealed the highest inhibitory (IC50 = 39.64 µM) (70). The essential oil of F. carduchorum has been also found to be of AChE inhibitory activity (IC50 = 23.6 µL mL-1) (34). In another study on AChE inhibitors via bioassay-guided isolation, the dichloromethane extract from the root of F. campestris was investigated. Three daucane ester derivatives (1-acetyl-5-angeloyl lapiferol, ferutinin, and siol anisate), two phenol derivatives (epielmanticine and 2-epilaserine), one polyacetylene (9-epoxyfalcarindiol), and three coumarin derivatives (coladin, coladonin, umbelliprenin) were isolated. All the obtained constituents could inhibit the AChE, but at higher doses (IC50 1.2–0.1 mM) than the standard galantamine (6.7 μM) and the most active compounds were the epielmanticine and the siol anisate with IC50 of 0.172 and 0.175 µM, respectively (82). In addition, Karakaya and co-workers studied the anticholinesterase effects of the fractions and extracts from the aerial parts and roots of F. isaurica and F. syriaca. Strong inhibitory activities against butyrylcholinesterase (BuChE) (88.56 ± 2.34%) and AChE (46.99%) at 20 µg/mL were observed in the CHCl3 part of the root of F. Isaurica. Felamidin (77.11%); prantschimgin (74.82%), two obtained constituents from the chloroform part of roots, presented a strong inhibitory effect against BuChE (19). In another study, AChE inhibitory activities of the volatile oils of the fruits, roots, and flowers of F. trifida were investigated. The result revealed significant AChE inhibitory activity (78.7, 74.3, and 72.1% inhibition of Ach Enzyme, respectively) (64). Hritcu and co-worker found the volatile oil extracted from the aerial part of F. angulata has an anti-amnesic activity in scopolamine-induced memory deterioration in rats and diminish AChE activity in hippocampal (99). In another study the volatile oil from fruits of F.pauciradiata revealed strong inhibitory properties against BuChE and AChE (IC50 = 0.567, 7.987 L/mL, respectively) (20).
Anti-diabetic effects
α-glucosidase, and α-amylase inhibitory activity of the extracts and some compounds from the roots of F. blancheana, F. trachycarpa, and F. pachyloba have been evaluated by in-vitro bioassay-guided isolation methods to determine the anti-diabetic properties of this plant. The obtained result demonstrated that the highest activities versus α-glucosidase with an IC50 value of 0.3, 2, 2 mg/mL belonged to CH2Cl2 extracts of the roots of F. trachycarpa, F. pachyloba, and F. blancheana, respectively. Suberosin and felamidin compounds possessed momentous α-glucosidase inhibitory activities with IC50 values of 0.9 and 0.4 mg/mL, respectively, while the IC50 for acarbose as standard was 4.9 mg/mL. The acarbose depicted a strong α-amylase inhibitory activity (82.3%) at a dose of 1 mg/mL whereas none of the other extracts displayed a significant α-amylase inhibitory effect (100).
Anti-coagulant activity
Golfakhrabadi et al. evaluated the toxicological profile of oral application of the F. carduchorum extract and the anticoagulant effects of two isolated coumarins (suberenol and suberosin) in male Wistar rats. The LD50 of the plant extract for acute toxicity was over 2000 mg/kg and there were no substantial variations (p > 0.05) among the control and the treated groups concerning the biochemical and hematological parameters. The prothrombin time (PT) of the treated group with a total extract from the aerial part of the plant has not shown a major impact relative to the control group (receiving tap water by gavage) (p > 0.05) at doses of 250 and 500 mg/kg. Meanwhile, suberosin expanded the PT at doses of 3 and 6 mg/kg (16.7 and 17.4 s, respectively) and suberenol at the same dose (16.5 and 17.1 s, respectively) (17).
Anti-malaria
Khanavi et al. assessed the larvicidal activity of chloroform, methanol, ethyl acetate, and the total 80% methanol extract from the aerial part of F. carduchorum against late 3rd, and early 4th instar larvae of the malaria vector Anopheles stephensi. The LC90 of chloroform, ethyl acetate, the total extract, and methanol fractions were 0.455, 1.892, 1.509, and 10.886 ppm, respectively. Moreover, the LC50 of chloroform, ethyl acetate, the total extract, and methanol fractions were 0.236, 0.744, 0.480, and 3.702 ppm, respectively. The chloroform fraction indicated lower LC50 and LC90 values than the other extracts, which might be due to the presence of high content of phytosteroids and coumarins (101). In another study, the larvicidal activity of a few isolated coumarin, methanol, and chloroform extracts of the roots, leaves, and fruits of F. trifida were investigated on the third instar larvae of A. stephensi Listonas. Strong insecticidal properties were found for methanol extract of the fruit with LC90 and LC50 values of 18.12 and 2.94 ppm, respectively. Among pure compounds, oxypeucedanin presented moderate toxicity against A. stephensi with LC90 and LC50 values of 346.41 and 116.54 ppm, respectively. It could be concluded that the methanol extract from the fruit of F. trifida might be utilized as an effective bio-insecticide in green control programs of mosquitoes, particularly A. stephensi (102).
Aphrodisiac activity
An in-vitro study was conducted by Ozturk et al. to investigate the aphrodisiac activity of the lyophilized water extract from the roots of F. syriaca on the human corpus cavernosum. For finding the mechanism involved in relaxation, the effect of the extract was investigated on the relaxing responses to selective guanilate cyclase inhibitor (oxadiazolo [4,3-α] quinoxalin-1one (ODQ)), NO-synthesis inhibitor (NG-nitro-L-arginine methyl ester (L-NAME)), forskolin, Electrical field stimulation (EFS), sodium nitroprusside, and acetylcholine. The result displayed that the extract could have to relax in a dose-dependent way on corpus cavernosum strips, and the L-NAME (small dosages) and QDQ (in all dosages) were able to suppress the extract-induced relaxation in the human corpus cavernosum. The F. syriaca extract enhanced the relaxation response of strips with Ach incubation while the extract did not affect the relaxation induced by EFS, forskolin sodium, and nitroprusside. The result suggested that the extract of F. syriaca could persumabely act via stimulating the NO- cyclic guanosine monophosphate (cGMP) pathway (103).
Toxicity profile of the genus Ferulago
Nowadays, the usage of herbal remedies has increased around the world, and patients falsely feel that they are healthy because they are natural. While these products comprise several bioactive compounds and might cause adverse effects on consumers (104, 105). Therefore, it is essential to herbal medicines undergo current safety and efficacy tests. Based on the literature review, there is only one piece of scientific study about the safety of this herb. Golfakhrabadi et al. investigated oral acute, and sub-chronic toxicities of the total extract of the aerial parts of F. carduchorum in the rat (17). To study acute toxicity five rats were treated with one dose of the total extract (2000 mg/kg) orally and the control group was treated with tap water. For the sub- chronic study, the doses (250, 500, and 1000 mg/kg) of the total extract were applied to treated groups by gavage for thirty following days. During the acute toxicity study, the animals did not show any signs of side effects, mortality, and the LD50 was over 2000 mg/kg in rats. The result of sub-chronic toxicity demonstrated that the total extract did not yield momentous changes in behavior, water, and food intake, breathing, body weight gain, blood metrics, and gastrointestinal properties in rats (17). This study indicated that F. carduchorum may be a healthy additive for conventional applications.
The accepted genus of Ferulago species based on The Plant List
| No. | Ferulago species | Synonyms |
|---|---|---|
| 1 | F. angulata (Schltdl.) Boiss. | F. angulata Schltdl., F. linearifolia Boiss., F. trifida Boiss. |
| 2 | F. angulata subsp. carduchorum (Boiss. & Hausskn.) D. F. Chamb | F. carduchorum Boiss. & Hausskn., F. abbreviata C. C. Towns., |
| 3 | F. antiochia Saya & Miski | |
| 4 | F. armena (DC.) Bernardi | F. pauciradiata Boiss. & Heldr., Ferula armena DC., F. bourgaei Boiss. Ferula gandogeri M.Hiroe. Ferula kochii M.Hiroe. F. sintenisii Gand. |
| 5 | F. aucheri Boiss. | Ferula taurica (Schlschk.) M. Hiroe, F. galbanifera var. brachyloba Boiss., F. taurica Schischk. |
| 6 | F. asparagifolia Boiss. | F. longistylis Boiss. |
| 7 | F. bernardii Tomk. & Pimenov | |
| 8 | F. biumbellata Pomel | Ferula sulcata var. biumbellata (Pomel), Ferula lutea var. mouretii (Maire) Maire. Batt. Ferula sulcata var. mouretii Maire |
| 9 | F. blancheana Post ex Boiss. | |
| 10 | F. bracteata Boiss. & Hausskn | |
| 11 | F. brachyloba Boiss. & Reut. | Ferula brachyloba (Boiss. & Reut.) Nyman |
| 12 | F. cassia Boiss. | F. amani Post ex Boiss. |
| 13 | F. contracta Boiss. & Hausskn | |
| 14 | F. daghestanica Schischk. | |
| 15 | F. fieldiana Rech. f. | Ferula fieldiana (Rech.f.) M. Hiroe |
| 16 | F. galbanifera (Mill.) W. D. J. Koch | Ferula daghestanica (Schischk.) M. Hiroe, Ferula campestris Besser, Ferula meoides L., Ferula galbaniifera Mill, F. campestris (Besser) Grecescu, F. meoides (L.) Boiss. F. dodonaei Kostel, |
| 17 | F. glareosa Kandemir & Hedge | |
| 18 | F. granatensis Boiss. | Ferula granatensis (Boiss.) Steud. |
| 19 | F. idaea Özhatay & Akalin | |
| 20 | F. humulis Boiss. | F. pumila Boiss. |
| 21 | F. isaurica Peşmen | |
| 22 | F. kurdica Post | |
| 23 | F. lutea (Poir.) Grande | Elaeoselinum mangenotianum Emb., Ferula capillaris Link ex Spreng., Ferula capillifolia Link., Ferula lutea (Poir.) Maire, Ferula lutea var. microcarpa (Maire)Maire, Ferula pomelii M.Hiroe, Ferula sulcata Desf., Ferula sulcata var. crassicosta (Pomel) Batt., Ferula sulcata var. leptocarpa (Pomel) Batt., Ferula sulcata var. microcarpa Maire, Ferula sulcata var. parvifolia (Pomel) Batt. F. barrelieri Guss., F. capillaris (Link ex Spreng.) Cout., F. capillifolia (Link) Franco, F. communis Petter ex Nyman, F. crassicosta Pomel, F. leptocarpa Pomel F. nodiflora Koch, F. parvifolia Pomel, F. sulcata (Desf.) Ledeb., Ligusticum luteum Poir. |
| 24 | F. macrocarpa (Fenzl) Boiss. | Ferula lophoptera (Boiss.) Benth. & Hook. f., Ferula mesopotamica, F. lophoptera Boiss. |
| 25 | F. macedonica Micevski & E. Mayer | |
| 26 | F. macrosciadea Boiss. & Balansa | |
| 27 | F. mughlae Peşmen | |
| 28 | F. nodosa (L.) Boiss. | Ferula cretica (Spreng.) M. Hiroe, Ferula geniculata Guss., Ferula nodosa (L.) Benth. & Hook. f., Ferula rigida Ten., Peucedanum nodosum L, F. geniculata Boiss. |
| 29 | F. pachyloba (Fenzl) Boiss. | Ferula cappadocica (Bornm.) M. Hiroe, Ferula pachyloba Fenzl,, F. cappadocica Bornm. |
| 30 | F. phialocarpa Rech. f. & Riedl | |
| 31 | F. sandrasica Peşmen & Quezel | |
| 32 | F. platycarpa Boiss. & Balansa | F. asperula Freyn & Sint |
| 33 | F. sartorii Boiss. | |
| 34 | F. scabra Pomel | Ferula algiersia M.Hiroe, Ferula sulcata var. scabra (Pomel) Batt. |
| 35 | F. serpentinica Rech.f. | |
| 36 | F. setifolia K. Koch | |
| 37 | F. stellata Boiss. | Ferula pauciflora K. Koch, Ferula setifolia K. Koch, Ferula sylvatica Szov. ex Boiss., F. oxyptera Boiss., F.pauciflora K. Koch |
| 38 | F. silaifolia (Boiss.) Boiss. | Ferula silaifolia (Boiss) M. Hiroe |
| 39 | F. subvelutina Rech. f | Ferula subvelutina (Rech. f.) M. Hiroe, Ferula turcomanica (Schischk.)M. Hiroe, F. turcomanica Schischk |
| 40 | F. sylvatica (Besser) Rchb. | Ferula barrelieri Ten., Ferula commutata (Racher) M. Hiroe, Ferula ferulago var. commutata Rochel, Ferula microphylla M.Bieb. ex Schur, Ferula monticola (Boiss. & Heldr.) Neilr., Ferula myriophylla M.Bieb. ex Besser, Ferula schischkinii M.Hiroe, Ferula sylvatica Besser, Ferula verticillata Maerkl. F. athamantifolia Schur, F. commutata (Rochel) Degen, F. latiloba Schischk., F. monticola Boiss. & Heldr., F. orphanidis Boiss. & Heldr., F. rocheliana Nyman, F. transsilvanica Schur |
| 41 | F. sylvatica subsp. confusa (Velen.) Hartvig | F. confuse Velen. |
| 42 | F. syriaca Boiss | F. cypria H.Wolff |
| 43 | F. ternatifolia Solanas, M.B.Crespo & García-Martín | |
| 44 | F. thirkeana Boiss | |
| 45 | F. trachycarpa Boiss. | Ferula insularis (H. Wolff) M. Hiroe, F. frigida Boiss., F. insularis H. Wolff |
| 46 | F. thyrsifolia (Sm.) Koch | Ferula thyrsifolia Sm. |
| 47 | F. trojana Akalın & Pi | |
| 48 | F. vesceritensis Coss. & Durieu ex Batt. |
| Plant name | Yield (%) | Methods | Part | Main components (%) | Class | Ref. |
|---|---|---|---|---|---|---|
| F. macedonica | - | HD | Inflorescence | α-Pinene (43.1), Sabinene (26.7) | Monoterpene (69.8) | (29) |
| - | Aerial parts | α-Pinene (22.8), Sabinene (15.5) | Monoterpene (38.3) | |||
| F. angulata | 2.8 | HD | Aerial parts | α-Pinene (15.4), cis-Ocimene (30) | Monoterpene (45.5) | (30) |
| 0.66 | Flowers | β-Phellandrene (16.5), α-Phellandrene (27), p-Cymene (10), α-Pinene (12) | Monoterpene (65.5) | (31) | ||
| 0.54 | Stems | β-Phellandrene (16), α-Phellandrene (18), p-Cymene (17.7), α-Pinene (21) | Monoterpene (72.7) | |||
| 0.43 | Leaves | α-Pinene (16.8), α-Phellandrene (20.7), p-Cymene (14.5), β-Phellandrene (16) | Monoterpene (68) | |||
| 2.65 | HD | Fruits | Limonene (38), α-Pinene (18.1) | Monoterpene (56.1) | (32) | |
| 6.5 | MAHD | Limonene (35), α-Pinene (14) | Monoterpene (49) | |||
| - | β-Phellandrene (32), α-Phellandrene (13.8) | Monoterpene (45.8) | (33) | |||
| F.carduchorum | 0.63 | HD | Leaves | α-Pinene (26), cis-Ocimene (24.5), Bornyl acetate (6.2) | Monoterpene (50.5), Oxygenated monoterpene (6.2) | (18) |
| 3.2 | Fruits | cis-Ocimene (64-76), α-Pinene (7.3-15) | Monoterpene (71.3-91) | |||
| 1.3 | HD | Aerial parts | (z)-β-Ocimene (43.5), α-Pinene (18.2) | Monoterpene (61.7) | (34) | |
| F. phialocarpa | 0.14 | HD | Aerial parts | α-Pinene (41), α-Phellandrene (14.2), β-Phellandrene (9.5) | Monoterpene (64.7) | (35) |
| 0.89 | Inflorescence | α-Pinene (43.38), cis-Chrysanthenyl acetate (6) 2,4,5-Trimethyl benzaldehyde (17). | Monoterpene (43.38), Oxygenated monoterpene (6) Oxygenated hydrocarbon (17) | (36) | ||
| F.contracta | 0.68 | HD | Aerial parts | β-Phellandrene (15), (E)-β-Ocimene(10) α-Phellandrene (14.5), β-eudesmol (11) | Monoterpene (39.5), Oxygenated sesquiterpene (11) | (37) |
| 1.3 | Flowers | β-Phellandrene (25), α-Phellandrene (25) | Monoterpene (50) | |||
| 0.54 | Leaves | β-Eudesmol (24.5 ), Spathulenol (16), Citronellol (12) | Oxygenated sesquiterpene (40.5), oxygenated monoterpene (12) | |||
| 0.4 | Stems | β-Phellandrene (15.5), α-Phellandrene (11.5) | Monoterpene (27) | |||
| F. macrocarpa | - | HD | Flowers | Bornyl acetate (37.1), Terpinolene (10) | Oxygenated monoterpene (37.1), Monoterpene (10) | (38) |
| - | Leaves | Bornyl acetate (37.91), o-Cymene (7.83) | Oxygenated monoterpene (37.91), Monoterpene (7.83) | |||
| - | Aerial parts | Bornyl acetate (45.7), Borneol (17.2) | Oxygenated monoterpene (62.9) | (39) | ||
| 0.03-0.04 | HD, MAHD | Flower | Borneol and Bornyl acetate | Oxygenated monoterpene | (40) | |
| 0.80 | HD | Fruit | Bornyl acetate (49), 2,3,6-Trimethyl benzaldehyde (7) | Oxygenated monoterpene (49), Oxygenated hydrocarbon (7) | (41) | |
| F. blancheana | - | HD | Flowers | Sabinene (23.2), Myrcene (17.5) | Monoterpene (40.7) | (42) |
| - | Aerial parts | Bornyl acetate (11.7), β-Caryophyllene (10.2) | Oxygenated monoterpene (11.7), Sesquiterpene (10) | |||
| - | Roots | E)-2-Decenal (20.3), Caryophyllene oxide (17.8) | Oxygenated hydrocarbon (20.3), Oxygenated sesquiterpene (17.8) | |||
| F. bernardii | 0.2 | HD | Aerial parts | 2,4,5-Trimethylbenzaldehyde (21), α-Pinene (17) | Oxygenated hydrocarbon (21), Monoterpene (17) | (10) |
| - | HD | Aerial parts | α-Pinene (35), Bornyl acetate(11.6), z-β-Ocimene (14.2) | Monoterpene (49.2), Oxygenated monoterpene (11.6) | (16) | |
| F. pachyloba | 1.5 | HD | Aerial parts | (Z)-β-Ocimene (25.7), α-Pinene (9.8) | Monoterpene (35.5) | (43) |
| F. longistylis | 0.16 | HD | Aerial parts | 2,3,6-trimethylbenzaldehyde (32.7), Bornyl acetate (12.6) | Oxygenated hydrocarbon (32.7), Oxygenated monoterpene (12.6) | (43) |
| 6.4 | Fruits | 2,3,6-Trimethylbenzaldehyde (29),(Z)-β-Ocimene (16), α-Pinene (17) | Oxygenated hydrocarbon (29), Monoterpene (33) | (7) | ||
| F. isaurica | 12 | HD | Fruits | α-Pinene (31.5), Limonene (24.2), Myrcene (17.0) | Monoterpene (72.7) | (44) |
| 0.7 | Roots | Terpinolene (42.1), Myrcene (27) | Monoterpene (69.1) | |||
| 0.08 | Aerial parts | Nonacosane (25.5), Hexadecanoic acid (14.8) | Hydrocarbon (25.5), Fatty acid (14.8) | (43) | ||
| F. syriaca | 4.8 | HD | Fruits | Myrcene (15.3), terpinolene (12.5), 4,6-Guaiadiene (10.7) | Monoterpene (27.8), Sesquiterpene (10.7) | (44) |
| 1.1 | Roots | Bornyl acetate (69.4), Terpinolene (12.5) | Oxygenated monoterpene (69.4), Monoterpene (12.5) | |||
| F. platycarpa | 0.07 | HD | Aerial parts | 2,3,6-Trimethylbenzaldehyde (29.8), cis-Chrysanthenyl acetate (24.2) | Oxygenated hydrocarbon (29.8), Oxygenated monoterpene (24.2) | (43) |
| F. thyrsiflora | 0.80 | HD | Aerial parts | Spathulenol (31) | Oxygenated sesquiterpene (31) | (45) |
| F. sylvatica | 0.10 | HD | Aerial parts | Sparhulenol (13) | Oxygenated sesquiterpene (13) | (45) |
| - | Roots | 2,3,6-Trimethylbenzaldehyde (92.7) | Oxygenated hydrocarbon (92.7) | (46) | ||
| - | Aerial parts | Germacrene D (32.5) | Sesquiterpene (32.5) | |||
| - | Inflorescence | Myrcene (29.2) | Monoterpene (29.2) | |||
| - | M.D | Fruits | p-Cymene (45.8), 2,5 dimethoxy p-Cymene (40) | Monoterpene (85.8) | (47) | |
| F. nodosa | 3 | HD | Aerial parts | α-Pinene (31) | Monoterpene (31) | (45) |
| - | SDE | 2,3,4-Trimethylbenzaldehyde (42.2), α-Pinene(22.5) | Oxygenated hydrocarbon (42.2), Monoterpene (22.5) | (48) | ||
| - | SFE | α-Pinene (55.5), Myrcene (10), cis-β-Ocimene (7) | Monoterpene (72.5) | |||
| F. pauciradiata | - | Fruits | trans-Chrysanthenyl acetate (25), 2,3,6-Trimethyl benzaldehyde (20.7), α-Pinene (23.7) | Oxygenated monoterpene (25), Oxygenated hydrocarbon (20.7) Monoterpene (23.7) | (20) | |
| - | HD | Fruits | Bornyl acetate (30.5), α-Pinene (7), Germacrene D (8) | Oxygenated monoterpene (30.5), Monoterpene (7), Sesquiterpene (8) | (49) | |
| - | Roots | 2,5, dimethoxy-p-Cymene (70), p-Cymene (12.5) | Monoterpene (82.5) | |||
| - | Aerial parts | 2,5, dimethoxy-p-Cymene (33), Nonacosane (9), α-Pinene (9) | Monoterpene (42), Hydrocarbon (9) | |||
| F.asparagifolia | - | M.D | Fruits | 2,3, 6-trimethylbenzaldehyde (42), α-Pinene (11) | Oxygenated hydrocarbon (42), Monoterpene (11) | (47) |
| - | HD | Fruits | 2, 3, 6-trimethylbenzaldehyde (38.9), Myrcene (18.2) | Oxygenated hydrocarbon (38.9), Monoterpene (18.2) | (50) | |
| F. aucheri | - | MD | Fruits | α-Pinene (36) | Monoterpene (36) | (47) |
| F. confuse | - | MD | Fruits | p-Cymene (24), 2,5-dimethoxy-p-Cymene (63.5) | Monoterpene (87.5) | (47) |
| F. galbanifera | - | MD | Fruits | trans-Chrysanthenyl acetate (17.2), Limonene (10), p-Cymene (12), α-Phellandrene (11), | Oxygenated monoterpene (17.2), Monoterpene (33) | (47) |
| 1.3 | HD | Fruits | α-Pinene (31.8), Sabinene (15.8), Limonene (7) | Monoterpene (54.6) | (51) | |
| F. humilis | MD | Fruits | Limonene (31), (Z)-b-Ocimene (32) | Monoterpene (63) | (47) | |
| 3.9 | HD | Fruits | Limonene (17.5), (Z)-β-Ocimene (32.5), α-Pinene (12) | Monoterpene (62) | (51) | |
| F. campestris | 0.11 | HD | Aerial parts | 2,4,5-trimethyl benzaldehyde, 2,4,6-trimethyl benzaldehyde | Oxygenated hydrocarbon | (52) |
| 0.13 | Flowers | |||||
| 0.05 | Roots | |||||
| 1.50 | Fruits | |||||
| 0.11 | MAHD | Aerial parts | ||||
| 0.13 | Flowers | |||||
| 0.10 | Roots | |||||
| 1.05 | Fruits | |||||
| HD | Roots | α-Pinene (58.3–75) | Monoterpene (58.3–75) | (23) | ||
| 6.4 | Fruits | Myrcene (36), α-Pinene (23), γ-Terpinene (10) | Monoterpene (69) | |||
| - | WD | Fruits | Myrcene (33.4–39.7), α-Pinene (23) | Monoterpene (56.4-62.7) | (53) | |
| F. idaea | - | MD | Fruits | p-Cymene (18.5), α-Pinene (16), Carvacrol methyl ether (13), 2,3,6-Trimethyl benzaldehyde (14) | Monoterpene (34.5), Oxygenated monoterpene (13), Oxygenated hydrocarbon (14) | (47) |
| F.macrosciadia | - | MD | Fruits | p-Cymene (19.5), Carvacrol methyl ether (78) | Oxygenated monoterpene (78), Monoterpene (19.5) | (47) |
| F. mughlae | - | MD | Fruits | α-Pinene (25.4), Cubenol (12.7) | Monoterpene (25.4), Oxygenated sesquiterpene (12.7) | (47) |
| - | HD | Aerial parts | α-Pinene (45.5), Camphene (10.5) | Monoterpene (56) | (54) | |
| - | Roots | α-Pinene (37.5), Borneol (9.5) | Monoterpene (37.5), Oxygenated monoterpene (9.5) | |||
| - | Fruits | α-Pinene (53), Myrcene (3.9), β-Phellandrene (11), Limonene (6) | Monoterpene (72.9) | |||
| F. sandrasica | - | MD | Fruits | α-Pinene (40.8), Germacrene D (8) | Monoterpene (40.8), Sesquiterpene (8) | (47) |
| - | HD | Herb | α-Pinene (26.5), Camphene (5), Caryophyllene oxide (6) | Monoterpene (31.5), Oxygenated sesquiterpene (6) | (55) | |
| - | Roots | α-Pinene (28), δ-3-Carene (14.2), Limonene (26) | Monoterpene (68.2) | |||
| 0.62 | Leaves | Ocimene (30.5), α-Pinene (17.8), δ-3-Carene (27.4) | Monoterpene (75.7) | (56) | ||
| 0.02 | HD | Aerial parts | Limonene (29), Terpinolene (14), α-Pinene (15.6) | Monoterpene (58.6) | (57) | |
| F. silaifolia | - | MD | Fruits | α-Pinene (5.5), Trans-Chrysanthenyl acetate (83.5) | Oxygenated monoterpene (83.5), Monoterpene (5.5) | (47) |
| F. trachycarpa | - | MD | Fruits | γ-Terpinene (27.8) | Monoterpene (27.8) | (47) |
| - | HD | Fruits | (Z)-β- Ocimene (34.1), α-Pinene (8) | Monoterpene (42.1) | (51) | |
| 7.3 | Fruits | (Z)-p-Ocimene (30.7), Myrcene (27.7) | Monoterpene (58.4) | (58) | ||
| F. thirkeana | 4.1 | HD | Fruits | Ferulagone (64), Germacrene D (14), α-Pinene (10) | Oxygenated monoterpene (64), Sesquiterpene (14), Monoterpene (10) | (59) |
| - | MD | Fruits | Ferulagone (56), Germacrene D (12), α-Pinene (9) | Oxygenated monoterpene (56), Sesquiterpene (12), Monoterpene (9) | ||
| F. setifolia | 0.26 | HD | Aerial parts | 2,4,5-Trimethyl benzaldehyde (77.8), 2,3,4-Trimethyl benzaldehyde (6.2) | Oxygenated hydrocarbon (84) | (28) |
| F. subvelutina | - | HD | Aerial parts | Limonene (27), α-Phellandrene (23.1), α-Pinene (13.3) | Monoterpene (63.4) | (60) |
| - | Aerial parts | Limonene (30), Terpinolene (14), α-Pinene (15.5), | Monoterpene (58.5) | (61) | ||
| F. stellata | 0.60 | HD | Aerial parts | 2,4,5-Trimethyl benzaldehyde (61.1) | Oxygenated hydrocarbon (61.1) | (60) |
| F.capillaris | - | HD | Aerial parts | α-Pinene (35.8), Limonene (30.9) | Monoterpene (66.7) | (62) |
| F. trifida | 1.5 | HD | Aerial parts | Isobornyl acetate (25.73), Verbenol (9.66), E-β-Caryophyllene (8.68) | Oxygenated monoterpene (35.39), Sesquiterpene (8.68) | (63) |
| 1.4 | Flowers | (E)-β-Ocimene (37.3), α-Pinene (16.5), Bornyl acetate (9.5) | Monoterpene (53.8), Oxygenated monoterpene (9.5) | (64) | ||
| 0.8 | Roots | Suberosin (20.7), Cuparene (6), β-Barbatene (6.5) | Coumarin (20.7), Sesquiterpene (12.5) | |||
| 1.3 | Stems | (E)-β-Ocimene (20.7), α-Pinene (22.6), Bornyl acetate (8.5) | Monoterpene (43.3), oxygenated monoterpene (8.5) | |||
| 1.6 | Leaves | (E)-β-Ocimene (25.7), Bornyl acetate (16.7), α-Pinene (19.6) | Monoterpene (45.3), Oxygenated monoterpene (16.7) | |||
| 1.4 | Fruits | (E)-β-Ocimene (30.5), Bornyl acetate (11), α-Pinene (18) | Monoterpene (48.5), Oxygenated monoterpene (11) |
Chemical compounds isolated from the Ferulago genus
Pharmacological activities and model of study of the genus Ferulago
| Pharmacological activities | Plant | Model | References |
|---|---|---|---|
| Anti-microbial | F. sandrasica | In-vitro | (55) |
| F. pachyloba, F. blancheana, F. trachycarpa, F. bracteata | In-vitro | (78) | |
| Anti-fungal | F. capillaris | In-vitro | (62) |
| Anti-oxidant | F. carduchorum | In-vitro | (97) |
| F. bernardii | In-vitro | (16) | |
| F. trifida, F. sandrasica, F.macedonica, | In-vitro | (64) | |
| F. cassia | In-vitro | (8) | |
| F. angulata, and F. campestris | In-vitro | (79) | |
| F. subvelutina | In-vitro | (74) | |
| Alzheimer | F. subvelutina, F. angulata | In-vitro | (98) |
| F.pauciradiata | In-vitro | (20) | |
| F. carduchorum | In-vitro | (34) | |
| F. isaurica and F. syriaca. | In-vitro | (19) | |
| F. trifida | In-vitro | (64) | |
| F. angulata | In-vitro | (99) | |
| F. pauciradiata | In-vitro | (20) | |
| Anti-diabetic | F. blancheana, F. pachyloba, F. trachycarpa | In-vitro | (100) |
| Anti-coagulant | F. carduchorum | In-vivo | (17) |
| Anti-malaria | F. carduchorum | In-vitro | (101) |
| F. trifida | In-vitro | (102) | |
| Aphrodisiac | F. syriaca | In-vitro | (103) |
Conclusion and future perspective
To date, around 73 molecules, including coumarin, furanocoumarin, flavonoids, and stilbene have been isolated from the Ferulago species. Among the isolated constituents, mostly are phenolic compounds in which three simple coumarins, sixteen furanocoumarins, five dihydro-furanocoumarin, four sesquiterpene coumarin, twelve prenylated coumarins, six pyranocoumarin, nine flavonoids, and eighteen miscellaneous compounds. Coumarins and their derivatives are considered to be important taxonomic markers of the genus Ferulago. In the pharmacological effect part, we have discussed various studies to evaluate the ethnomedicinal uses and this has shown that the isolated constituents and different extracts from the Ferulago species possess several pharmacological effects including antibacterial, antioxidant, Alzheimer, anti-diabetics, anti-malaria, anti-coagulant, aphrodisiac effects. Accessible information regarding Ferulago spp. allows us to explore their potential benefits, highlight the gaps in our knowledge, and conduct future researches to develop new drugs. It can be concluded that the pharmacological study of Ferulago spp. is in agreement with the ethnomedicinal uses of the plants.
Acknowledgements
References
-
1.
Salehi B, Krochmal‐Marczak B, Skiba D, Patra JK, Das SK, Das G, Popović‐Djordjević JB, Kostić AŽ, Anil Kumar NV, Tripathi A. Convolvulus plant—A comprehensive review from phytochemical composition to pharmacy. Phytother. Res. 2019;34:315-28. [PubMed ID: 31713286].
-
2.
Tamokou J, Mbaveng A, Kuete V. Antimicrobial activities of African medicinal spices and vegetables. In: Kuete V, editor. Medicinal spices and vegetables from Africa. 1nd ed. Academic Press, Elsvier Inc. 2017:207-37.
-
3.
Amiri MS, Joharchi MR. Ethnobotanical knowledge of Apiaceae family in Iran: A review. Avicenna J. Phytomed. 2016;6:621. [PubMed ID: 28078243].
-
4.
Sahebkar A, Iranshahi M. Biological activities of essential oils from the genus Ferula (Apiaceae). Asian Biomed. 2010;4:835-47.
-
5.
Lorigooini Z, Koravand M, Haddadi H, Rafieian-Kopaei M, Shirmardi HA, Hosseini Z. A review of botany, phytochemical and pharmacological properties of Ferulago angulata. Toxin Rev. 2019;38:13-20.
-
6.
Karakaya S, Simsek D, Göger G, Demirci B, Duman H, Altanlar N, Kiliç CS. Comparison of essential oils of Ferulago pachyloba (Fenzl) Boiss F trachycarpa Boiss and F bracteata Boiss & Hausskn species (Apiaceae) growing in Turkey and determination of their antimicrobial activities. J. Essent. Oil Bear. Plants. 2019;22:200-13.
-
7.
Özkan AMG, Demirci B, Demirci F, Başer KHC. Composition and antimicrobial activity of essential oil of Ferulago longistylis Boiss. fruits. J. Essent. Oil Res. 2008;20:569-73.
-
8.
Karakaya S, Koca M, Sytar O, Dursunoglu B, Ozbek H, Duman H, Guvenalp Z, Kılıc C. Antioxidant and anticholinesterase potential of Ferulago cassia with farther bio-guided isolation of active coumarin constituents. S. Afr. J. Bot. 2019;121:536-42.
-
9.
Urusak EA, Kizilarslan Ç. Fruit anatomy of some Ferulago (Apiaceae) species in Turkey. Turk. J. Bot. 2013;37:434-45.
-
10.
Khalighi-Sigaroodi F, Hadjiakhoondi A, Shahverdi AR, Mozaffarian V-A, Shafiee A. Chemical composition and antimicrobial activity of the essential oil of Ferulago bernardii Tomk and M. Pimen. Daru J. Pharm. Sci. 2005;13:100-4.
-
11.
-
12.
Shahbazi Y, Shavisi N, Karami N, Kakaei S. Chemical composition and in vitro antibacterial activity of Ferulago angulata (Schlecht. Boiss essential oil. Pharm. Sci. 2015;21:6.
-
13.
Javidnia K, Miri R, Edraki N, Khoshneviszadeh M, Javidnia A. Constituents of the volatile oil of Ferulago angulata (Schlecht. Boiss. from Iran. J. Essent. Oil Res. 2006;18:548-50.
-
14.
Dikpınar T, Süzgeç-Selçuk S, Çelik BÖ, Uruşak EA. Antimicrobial activity of rhizomes of Ferulago trachycarpa Boiss and bioguided isolation of active coumarin constituents. Ind. Crops Prod. 2018;123:762-7.
-
15.
Hayta S, Dogan G, Demirpolat A, Bagci E. Identification of essential oil composition of four umbelliferae from Turkey. Nat. Sci. Discov. 2015;1:74-9.
-
16.
Shahbazi Y, Shavisi N. Chemical composition, antioxidant and antimicrobial activities of the essential oil and methanolic extract of Ferulago bernardii Tomk M. Pimen of Iran. Arch. Phytopathol. Plant Prot. 2015;48:699-710.
-
17.
Golfakhrabadi F, Abdollahi M, Ardakani MRS, Saeidnia S, Akbarzadeh T, Ahmadabadi AN, Ebrahimi A, Yousefbeyk F, Hassanzadeh A, Khanavi M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 2014;52:1335-40. [PubMed ID: 25017518].
-
18.
Ghasempour H, Shirinpour E, Heidari H. The constituents of essential oils of Ferulago angulata (Schlecht ) Boiss at two different habitats, Nevakoh and Shahoo, Zagross Mountain, western Iran. Iran. J. Sci. Technol. Trans. A Sci. 2007;31:309-12.
-
19.
Karakaya S, Koca M, Kılıc CS, Coskun M. Antioxidant and anticholinesterase activities of Ferulago syriaca Boiss and F isaurica Peșmen growing in Turkey. Med. Chem. Res. 2018;27:1843-50.
-
20.
Karakaya S, Koca M, Simsek D, Bostanlik FD, Özbek H, Kiliç CS, Güvenalp Z, Demirci B, Altanlar N. Antioxidant, Antimicrobial and Anticholinesterase Activities of Ferulago pauciradiata Boiss & Heldr Growing in Turkey. J. Biol. Act. Prod. Nat. 2018;8:364-75.
-
21.
Ahmad SA, Askari AA. Ethnobotany of the Hawraman region of Kurdistan Iraq. Harv. Pap. Bot. 2015;20:85-9.
-
22.
Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4:58.
-
23.
Cecchini C, Coman MM, Cresci A, Tirillini B, Cristalli G, Papa F, Sagratini G, Vittori S, Maggi F. Essential oil from fruits and roots of Ferulago campestris (Besser) Grecescu (Apiaceae): composition and antioxidant and anti‐Candida activity. Flavour Fragr. J. 2010;25:493-502.
-
24.
Périno-Issartier S, Ginies C, Cravotto G, Chemat F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A. 2013;1305:41-7. [PubMed ID: 23890545].
-
25.
Sodeifian G, Ansari K. Optimization of Ferulago Angulata oil extraction with supercritical carbon dioxide. J. Supercrit. Fluids. 2011;57:38-43.
-
26.
Maxia A, Marongiu B, Piras A, Porcedda S, Tuveri E, Gonçalves MJ, Cavaleiro C, Salgueiro L. Chemical characterization and biological activity of essential oils from Daucus carota L subsp carota growing wild on the Mediterranean coast and on the Atlantic coast. Fitoterapia. 2009;80:57-61. [PubMed ID: 18950693].
-
27.
Taran M, Ghasempour HR, Shirinpour E. Antimicrobial activity of essential oils of Ferulago angulata subsp Carduchorum. Jundishapur J. Microbiol. 2010:10-4.
-
28.
Polat T, Özer H, Mete E, Çakir A, Kandemir A, Özturk E. Essential oil composition of Ferulago setifolia C Koch growing wild in Eastern Anatolia, Turkey. J. Essent. Oil-Bear. Plants. 2010;13:20-4.
-
29.
Mileski KS, Dzamic AM, Ciric AD, Ristic MS, Grujic SM, Matevski VS, Marin PD. Composition, antimicrobial and antioxidant properties of endemic species Ferulago macedonica Micevski & E. Mayer. Rec. Nat. Prod. 2015;9:208.
-
30.
Khanahmadi M, Janfeshan K. Study on antioxidation property of Ferulago angulata plant. Asian J. Plant Sci. 2006;5:521-6.
-
31.
Akhlaghi H. The essential oils from flowers, stems and leaves of Ferulago angulata from Iran. Chem. Nat. Compd. 2008;44:396-7.
-
32.
Mollaei S, Sedighi F, Habibi B, Hazrati S, Asgharian P. Extraction of essential oils of Ferulago angulata with microwave-assisted hydrodistillation. Ind. Crops Prod. 2019;137:43-51.
-
33.
Rustaiyan A, Sedaghat S, Larijani K, Khossravi M, Masoudi S. Composition of the essential oil of Ferulago angulata (Schlecht). Boiss. from Iran. J. Essent. Oil Res. 2002;14:447-8.
-
34.
Golfakhrabadi F, Khanavi M, Ostad SN, Saeidnia S, Vatandoost H, Abai MR, Hafizi M, Yousefbeyk F, Rad YR, Baghenegadian A. Biological activities and composition of Ferulago carduchorum essential oil. J. Arthropod-Borne Dis. 2015;9:104.
-
35.
Masoudi S, Rustaiyan A, Ameri N. Volatile oils of Ferulago phialocarpa Rech et H Reidl and Leutea elbursensis Mozaffarian from Iran. J. Essent. Oil Res. 2004;16:143-4.
-
36.
Ghorbanpour M, Hosseini N, Rad AM. Chemical Composition of the Essential Oil of Ferulago phialocarpa Rech Riedl An Endemic Medicinal Plant from Iran. J. Essent. Oil Bear. Plants. 2016;19:778-81.
-
37.
Mohebat R, Mosslemin MH, Masoudi S, Dad V, Rustaiyan A. Composition and antibacterial activity of the essential oils from aerial parts, stems, flowers and leaves of Ferulago contracta from Iran. J. Essent. Oil Bear. Plants. 2010;13:607-14.
-
38.
Azarbani F, Jafari S, Shiravand S. Phytochemicals, phenolic profiles, antioxidant and antibacterial activities of Ferulago macrocarpa Extracts from Lorestan. J. Medicinal plants and By-product. 2019;8:125-32.
-
39.
Hadjiakhoondi A, Aghel N, Etemadi R. Chemical and biological study of essential oil of Ferulago macrocarpa (Fenzi) Boiss. Hamdard Med. 2002;45:35-7.
-
40.
Asghari J, Salehi M, Mazaheritehrani M. Isolation of borneol and bornyl acetate from Ferulago macrocarpa by microwave irradiation. J. Herb. Med. 2013;4:89-93.
-
41.
Sajjadi S, Shokoohinia Y, Jamali M. Chemical composition of essential oil of Ferulago macrocarpa (Fenzl) Boiss. fruits. Res. Pharm. Sci. 2012;7:197. [PubMed ID: 23181098].
-
42.
Karakaya S, Göger G, Kılıç CS, Demirci B. Composition of volatile oil of the aerial parts, flowers and roots of Ferulago blancheana Post.(Apiaceae) growing in Turkey and determination of their antimicrobial activities by bioautography method. Turk. J. Pharm. Sci. 2016;13:173-80.
-
43.
Kılıç CS, Özkan AMG, Demirci B, Coşkun M, Başer KHC. Essential oil composition of four endemic Ferulago species growing in Turkey. Nat. Prod. Commun. 2010;5:1934578X1000501225.
-
44.
Erdurak C, Coşkun M, Demirci B, Başer K. Composition of the essential oil of fruits and roots of Ferulago isaurica Peşme syriaca Boiss (Umbelliferae) from. Turkey. Flavour Fragr. J. 2006;21:118-21.
-
45.
Demetzos C, Perdetzoglou D, Gazouli M, Tan K, Economakis C. Chemical analysis and antimicrobial studies on three species of Ferulago from Greece. Planta Medica. 2000;66:560-3. [PubMed ID: 10985086].
-
46.
Stamenković JG, Petrović GM, Jovanović OP, Ickovski JD, Palić IR, Stojanović GS. Chemical composition of the essential oils and headspace volatiles of Ferulago sylvatica (Besser) Reichenb. from Serbia. Nat. Prod. Res. 2019:1-4.
-
47.
Baser KHC, Demirci B, Özek T, Akalin E, Özhatay N. Micro-distilled volatile compounds from Ferulago species growing in western Turkey. Pharm. Biol. 2002;40:466-71.
-
48.
Ruberto G, Biondi D, Renda A. The composition of the volatile oil of Ferulago nodosa obtained by steam distillation and supercritical carbon dioxide extraction. Phytochem. Anal. 1999;10:241-6.
-
49.
Cumhur B, Kırcı D, Kılıç C, Demirci B, Duman H. Essential oil composition of roots, aerial parts and fruits of Ferulago pauciradiata Boiss. & Heldr. Planta Medica. 2019;85:P-158.
-
50.
Baser KHC, Demirci B, Duman H. Composition of the essential oil of Ferulago asparagifolia Boiss from Turkey. J. Essent. Oil Res. 2001;13:134-5.
-
51.
Demirci F, İşcan G, Güven K, Başer KHC. Antimicrobial activities of Ferulago Essential Oils. Zeitschrift für Naturforschung C. 2000;55:886-9.
-
52.
Riela S, Bruno M, Rosselli S, Saladino ML, Caponetti E, Formisano C, Senatore F. A study on the essential oil of Ferulago campestris: How much does extraction method influence the oil composition? J. Sep. Sci. 2011;34:483-92.
-
53.
Sabbieti MG, Agas D, Maggi F, Vittori S, Marchetti L. Molecular mediators involved in Ferulago campestris essential oil effects on osteoblast metabolism. J. Cell. Biochem. 2011;112:3742-54. [PubMed ID: 21815199].
-
54.
Karakaya S, Delimustafaoglu Bostanlik F, Goger G, Demirci B, Kilic C. Comparison of essential oils and antimicrobial activities of Ferulago mughlae Peșmen (Apiaceae) growing in Turkey. J. Res. Pharm. 2019;23:76-82.
-
55.
Melike S, Kırcı D, Demirci B, Duman H, Kılıç CS, İlhan G. In vitro antimicrobial evaulation of Ferulago sandrasica Peșmen & Quézel herba and root essential oil. Nat. Volatiles Essent. Oils. 2019;6:13-20.
-
56.
Celik A, Arslan I, Herken EN, Ermis A. Constituents, oxidant-antioxidant profile, and antimicrobial capacity of the essential oil obtained from Ferulago sandrasica Peşmen and Quézel. Int. J. Food Prop. 2013;16:1655-62.
-
57.
Karakaya S, Göğer G, Bostanlik FD, Demirci B, Duman H, Kılıç CS. Comparison of the Essential Oils of Ferula orientalis L Ferulago sandrasica Pesmen and Quezel, and Hippomarathrum microcarpum Petrov and their antimicrobial activity. Turkish J. Pharm. Sci. 2019;16:69.
-
58.
Baser K, Koyuncu M, Vural M. Composition of the essential oil of Ferulago trachycarpa (Fenzl) Boiss. J. Essent. Oil Res. 1998;10:665-6.
-
59.
Başer KH, Demirci B, Demirci F, Hashimoto T, Asakawa Y, Noma Y. Ferulagone: A new monoterpene ester from Ferulago thirkeana essential oil. Planta medica. 2002;68:564-7. [PubMed ID: 12094309].
-
60.
Masoudi S, Eghbali H, Abadi VK. Volatile constituents of the aerial parts of Ferulago subvelutina Rech Ferulago stellata Boiss leaves and flowers of Prangos ferulacea (L ) Lindle and leaves of Ferula ovina (Boiss ) Boiss Four Umbelliferae herbs from Iran. J. Essent. Oil Bear. Plants. 2016;19:592-605.
-
61.
Chalabian F, Monfared A, Larijani K, Saldouzi S. Comparison of the essential oils of Chenopodium botrys L Ferulago subvelutina Rech Rosa gallica L and antimicrobial activity of the oils against some microbes. Iran. J. Med. Aromatic Plants. 2006;22:146-54.
-
62.
Pinto E, Hrimpeng K, Lopes G, Vaz S, Gonçalves M, Cavaleiro C, Salgueiro L. Antifungal activity of Ferulago capillaris essential oil against Candida, Cryptococcus, Aspergillus and dermatophyte species. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:1311-20. [PubMed ID: 23619574].
-
63.
Tavakoli S, Vatandoost H, Zeidabadinezhad R, Hajiaghaee R, Hadjiakhoondi A, Abai MR, Yassa N. Gas chromatography, GC/mass analysis and bioactivity of essential oil from aerial parts of Ferulago trifida: antimicrobial, antioxidant, AChE inhibitory, general toxicity, MTT assay and larvicidal activities. J. Arthropod-Borne Dis. 2017;11:414.
-
64.
Tavakoli S, Yassa N, Delnavazi M, Akhbari M, Hadjiakhoondi A, Hajimehdipoor H, Khalighi-Sigaroodi F, Hajiaghaee R. Chemical composition and biological activities of the essential oils from different parts of Ferulago trifida Boiss. J. Essent. Oil Res. 2017;29:407-19.
-
65.
Jiménez B, Grande MaC, Anaya J, Torres P, Grande M. Coumarins from Ferulago capillaris and F brachyloba. Phytochemistry. 2000;53:1025-31. [PubMed ID: 10820825].
-
66.
Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed. Res. Int. 2013:2013.
-
67.
Doganca S, Tuzlaci E, Ulubelen A. Constituents of Ferulago asperigifolia. Fitoterapia-Milano. 1992;63:552.
-
68.
Khalighi-Sigaroodi F, Hadjiakhoondi A, Shafiee A, Mozaffarian V, Shahverdi A, Alavi S. Phytochemical analysis of Ferulogo bernardii Tomk & M. pimen. DARU J. Pharm. Sci. 2006;14:214-21.
-
69.
Tavakoli S, Delnavazi M-R, Hadjiaghaee R, Jafari-Nodooshan S, Khalighi-Sigaroodi F, Akhbari M, Hadjiakhoondi A, Yassa N. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss an endemic species to Iran. Nat. Prod. Res. 2018;32:2724-8. [PubMed ID: 28954543].
-
70.
Golfakhrabadi F, Ardakani MRS, Saeidnia S, Akbarzadeh T, Yousefbeyk F, Jamalifar H, Khanavi M. In vitro antimicrobial and acetylcholinesterase inhibitory activities of coumarins from Ferulago carduchorum. Med. Chem. Res. 2016;25:1623-9.
-
71.
Serkerov S, Kagramanov A, Abbasov R. Coumarins of Ferulago turcomanica. Chem. Nat. Compd. 1976;12:82.
-
72.
Ognyanov I, Bocheva D. Natural Comarins. Planta Medica. 1969;17:65-70.
-
73.
De Pascual T, Jimenez B, Corrales B, Grande M. Coumarins from Ferulago granatensis group: isovaleryl marmesin. An. Quim. 1979.
-
74.
Naseri M, Monsef-Esfehani H, Saeidnia S, Dastan D, Gohari A. Antioxidative coumarins from the roots of Ferulago subvelutina. Asian J. Chem. 2013;25:1875-8.
-
75.
Satir E, Kilic C, Coskun M. Prantschimgin content of methanolic extract of roots of Ferulago platycarpa. Chem. Nat. Compd. 2009;45:872-3.
-
76.
Sklyar YE, Andrianova V, Pimenov M. Coumarins of the roots of Ferulago sylvatica. Chem. Nat. Compd. 1982;18:488.
-
77.
Ameen BAH. Phytochemical study and cytotoxic activity of Ferulago angulata (Schlecht) Boiss, from Kurdistan-region of Iraq. Int. J. Innov. Res. Adv. Eng. 2014;1:1-5.
-
78.
Karakaya S, Şimşek D, Özbek H, Güvenalp Z, Altanlar N, Kazaz C, Kılıc C. Antimicrobial activities of extracts and isolated coumarins from the roots of four Ferulago species growing in Turkey. Iran. J. Pharm. Res. 2019;18:1516-29. [PubMed ID: 32641960].
-
79.
Basile A, Sorbo S, Spadaro V, Bruno M, Maggio A, Faraone N, Rosselli S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules. 2009;14:939-52. [PubMed ID: 19255552].
-
80.
Doganca S, Ulubelen A, Tuzlaci E. 1-Acetylhydroquinone 4-galactoside from Ferulago aucheri. Phytochemistry. 1991;30:2803-5.
-
81.
Alkhatib R, Hennebelle T, Roumy V, Sahpaz S, Süzgeç S, Akalın E, Meriçli A, Bailleul F. Coumarins, caffeoyl derivatives and a monoterpenoid glycoside from Ferulago asparagifolia. Biochem. Syst. Ecol. 2009;37:230-3.
-
82.
Dall’Acqua S, Maggi F, Minesso P, Salvagno M, Papa F, Vittori S, Innocenti G. Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae). Fitoterapia. 2010;81:1208-12. [PubMed ID: 20713133].
-
83.
Dall’Acqua S, Linardi MA, Maggi F, Nicoletti M, Petitto V, Innocenti G, Basso G, Viola G. Natural daucane sesquiterpenes with antiproliferative and proapoptotic activity against human tumor cells. Bioorg. Med. Chem. 2011;19:5876-85. [PubMed ID: 21885290].
-
84.
Genovese S, Taddeo VA, Epifano F, Fiorito S, Bize Cc, Rives A, de Medina P. Characterization of the degradation profile of umbelliprenin, a bioactive prenylated coumarin of a Ferulago species. J. Nat. Prod. 2017;80:2424-31. [PubMed ID: 28853883].
-
85.
Taddeo VA, Epifano F, Preziuso F, Fiorito S, Caron N, Rives A, de Medina P, Poirot M, Silvente-Poirot S, Genovese S. HPLC analysis and skin whitening effects of umbelliprenin-containing extracts of Anethum graveolens, Pimpinella anisum, and Ferulago campestris. Molecules. 2019;24:501.
-
86.
Dall’Acqua S, Linardi MA, Bortolozzi R, Clauser M, Marzocchini S, Maggi F, Nicoletti M, Innocenti G, Basso G, Viola G. Natural daucane esters induces apoptosis in leukaemic cells through ROS production. Phytochemistry. 2014;108:147-56. [PubMed ID: 25294094].
-
87.
Sadeghi E, Ebadi S, Karami F, Bashiry M, Sharafi H. Evaluation of antimicrobial effect of Ferulago angulata essential oil and Nisin on Staphylococcus aureus growth in Iranian white cheese production and preservation trend. Int. J. Pharm. Technol. 2016;8:17927-41.
-
88.
Sajjadi S-E, Jamali M, Shokoohinia Y, Abdi G, Shahbazi B, Fattahi A. Antiproliferative evaluation of terpenoids and terpenoid coumarins from Ferulago macrocarpa (Fenzl) Boiss. fruits. Pharmacogn. Res. 2015;7:322.
-
89.
Jyoti M, Assenov I. Phytochemical studies on Ferulago sylvatica. Fitoterapia (Milano). 1995;66:88-9.
-
90.
Khanahmadi M, Shahrezaei F, Alizadeh A. Isolation and structural elucidation of two flavonoids from Ferulago angulata (Schlecht) Boiss. Asian J. Res. Chem. 2011;4:1667-70.
-
91.
Doğanca S, Hırlak F, Tüzün OT, Gürkan E. Phytochemical investigation on Ferulago confusa. J. Fac. Pharm. Istanbul Univ. 1995;31:41-6.
-
92.
Karimian H, Moghadamtousi SZ, Fadaeinasab M, Golbabapour S, Razavi M, Hajrezaie M, Arya A, Abdulla MA, Mohan S, Ali HM. Ferulago angulata activates intrinsic pathway of apoptosis in MCF-7 cells associated with G1 cell cycle arrest via involvement of p21/p27. Drug Des. Devel. Ther. 2014;8:1481.
-
93.
Miski M, Moubasher HA, Mabry TJ. Sesquiterpene aryl esters from Ferulago antiochia. Phytochemistry. 1990;29:881-6.
-
94.
Ahmadi F, Sadeghi S, Modarresi M, Abiri R, Mikaeli A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth of Iran. Food Chem. Toxicol. 2010;48:1137-44. [PubMed ID: 20132856].
-
95.
Asgharian P, Afshar FH, Asnaashari S, Lotfipour F, Baradaran B. Evaluation of various biological activities of the aerial parts of Scrophularia frigida growing in Iran. Iran. J. Pharm. Res. 2017;16:277. [PubMed ID: 28496481].
-
96.
Formisano C, Oliviero F, Rigano D, Saab AM, Senatore F. Chemical composition of essential oils and in vitro antioxidant properties of extracts and essential oils of Calamintha origanifolia and Micromeria myrtifolia, two Lamiaceae from the Lebanon flora. Ind. Crop. Prod. 2014;62:405-11.
-
97.
Golfakhrabadi F, Ardekani MRS, Saeidnia S, Yousefbeyk F, Jamalifar H, Ramezani N, Akbarzadeh T, Khanavi M. Phytochemical analysis, antimicrobial, antioxidant activities and total phenols of Ferulago carduchorum in two vegetative stages (flower and fruit). Pak. J. Pharm. Sci. 2016;29:623-8. [PubMed ID: 27087085].
-
98.
Hajimehdipoor H, Shekarchi M, Aghighi A, Hamzeloo-Moghadam M. Evaluating the acetylcholinesterase inhibitory activity of Ferulago angulate and Ferulago subvelutina. Res. J. Pharmacogn. 2014:39-43.
-
99.
Hritcu L, Bagci E, Aydin E, Mihasan M. Antiamnesic and antioxidants effects of Ferulago angulata essential oil against scopolamine-induced memory impairment in laboratory rats. Neurochem. Res. 2015;40:1799-809. [PubMed ID: 26168780].
-
100.
Karakaya S, Özbek H, Gözcü S, Güvenalp Z, Yuca H, Duman H, Kazaz C, Kiliç CS. α-Amylase and α-glucosidase inhibitory activities of the extracts and constituents of Ferulago blancheana pachyloba and trachycarpa roots. Bangladesh J. Pharmacol. 2018;13:35-40.
-
101.
Khanavi M, Baghernezhadian A, GolFakhrabadi F, Abai M, Vatandoost H, Hadjiakhoondi A. Larvicidal activity of Ferulago carduchorum Boiss & Hausskn against the main malaria vecto Anopheles stephensi. Res. J. Pharmacogn. 2016;3:19-22.
-
102.
Goodarzi S, Tavakoli S, Abai MR, Amini Z, Vatandoost H, Yassa N, Hadjiakhoondi A, Tofighi Z. Strong insecticidal potential of methanol extract of Ferulago trifida fruits against Anopheles stephensi as malaria vector. Environ. Sci. Pollut. Res. 2019;26:7711-7.
-
103.
Oztürk B, Gür S, Coskun M, Kosan M, Erdurak C, Hafez G, Ozgunes O, Cetinkaya M. 237 Relaxant effect of Ferulago syriaca root extract on human corpus cavernosum. Eur. Urol. Suppl. 2004;3:62.
-
104.
Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am. J. Med. 2004;116:478-85. [PubMed ID: 15047038].
-
105.
Abotsi WK, Ainooson GK, Gyasi EB, Abotsi WKM. Acute and sub-acute toxicity studies of the ethanolic extract of the aerial parts of Hilleria latifolia (Lam) H Walt(Phytolaccaceae) in rodents. West African J. Pharm. 2011;22:27-35.