Abstract
Keywords
Procalcitonin Colistin Critically ill patients Dosing interval MDR infections
Introduction
Inappropriate antibiotic therapy including wrong doses and/or duration of therapy in patients with intensive care unit (ICU) acquired infections, is associated with high mortality and morbidity which has been demonstrated in multiple studies (1-3).
On the other hand, excessive use of broad-spectrum antibiotics is associated with the development of resistant strains of different microorganisms, longer length of stay in ICU and hospital (1).
The increasing prevalence of infections caused by multi drug-resistant (MDR) gram-negative bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii have become a serious health problem worldwide (2). This has in turn led to the reintroduction of older antibiotics, such as colistin, with their potential adverse reactions and strong tendency for development of the newer resistant strains(3-5)
Colistin is a cationic polypeptide antimicrobial agent with a narrow bactericidal spectrum of activity against gram negative bacteria including P.aeruginosa, Acinetobacter spp. and Klebsiella spp.(6, 7). This agent is frequently administered in critically-ill patients suffering from (MDR) gram-negative infections (8-10). The available injectable formulation of colistin is penta-sodium colistin methane sulfonate or colistimethate sodium (CMS) which is an inactive prodrug with lesser potency and toxicity than colistin sulfate (11).
In critically ill patients, the existing colistin dosing schedules may result in suboptimal peak levels corresponding to the (MIC) break points of MDR gram-negative bacteria, leading to inappropriate delays in effective management of these microorganisms. Consequently, different approaches such as administration of CMS in higher doses and using extended dosing-intervals, have been suggested to achieve a profile (12-17).
In order to guide antimicrobial administration more efficiently and to prevent their overuse, several serum biomarkers have been studied in different clinical settings. One such biomarker is procalcitonin (PCT) which has been studied extensively over the past two decades as a serum marker of systemic infection and sepsis.
Circulating procalcitonin is a peptide of 114 amino acids, lacking the N-terminal dipeptide alanine-proline. In addition to the calcium homeostasis, procalcitonin play pivotal roles in the metabolic and inflammatory host response to microbial infections.(18, 19)
Five systematic reviews have evaluated the role of PCT in managing antibiotic administration in critically ill patients (20-24). All of them come to a comparable deduction that PCT can reduce the duration of antibiotics use by around 2–3 days, without any significant effect on mortality or the rates of reinfection.
In a study, Crain et al demonstrated that employment of (PCT), as a tool for antibiotics de-escalation led to significant reduction (55%) of antibacterial use in patients with severe community-acquired pneumonia (25).Stolz et al. on the other hand showed that the rates of hospitalization due to acute exacerbations of chronic obstructive pulmonary disease was not significantly different between the (PCT) guided and control groups during 6 months follow up (26).
The aim of our study was to evaluate the PCT changes in the two different high-dose colistin regimens, used for the management of MDR gram negative infections in ICU patients. The rationale of our study was based upon the pharmacokinetics and pharmacodynamics of this agent, where its administration in the form of once daily infusion could have superior bactericidal properties, hence more favorable impact on PCT reduction in this patient group.
Method
This is a prospective, randomized-controlled trial performed in the general ICU of a 550 bed university hospital in Tehran, Iran, from 2014 to 2015. The Ethics Committee of the Shahid Beheshti University, Tehran, Iran approved the study protocol. The study was registered in Iranian Registry of Clinical Trials. (Registration date: July 10, 2014, Number: IRCT2014062510178N7).
Adult ICU patients with the diagnosis of bacteremia and ventilator associated pneumonia (VAP) with MDR gram negative organisms were included in the study. (VAP) was defined according to the criteria of the American Thoracic Society Consensus Conference on (VAP) (27) .Bacteremia was defined by microbial growth in one blood culture bottle (28). MDR organisms were defined based on the ECDC/CDC characterization as those organisms with resistance to at least one agent in three or more antimicrobial classes (29).
Colistin was prescribed as CMS (Colomycin; Forest Laboratories, United Kingdom) prepared in 100-mL sterile isotonic saline and was infused over 30 minutes. Using permuted box randomization, patients were randomly assigned to group A and B. Patients in group A received CMS 9 million IU once daily and patients in group B received CMS 3 million IU every 8 h.
Inclusion criteria were age >18 years, and documented MDR infections. The exclusion criteria were pregnancy, breastfeeding, body mass index of over 35 Kg/m2, and duration of colistin treatment less than 3 days. Serum PCT levels were measured at the start, 3rd and 5th days of the CMS therapy and their differences between the two dose intervals were compared.
The measurements of PCT samples were made by electrochemiluminescence immunoassay using the COBAS e 411 equipment (Hoffmann-La Roche, Inc, Basel, Switzerland). A serum (PCT levels) of 0.1 µg/L or less indicated the lack of infection and led to the strong recommendation against antibiotics use. PCT levels of 0.1-0.25 µg/L suggested bacterial infection to be unlikely, 0.25- 0.5 µg /L suggested bacterial infection to be possible and 0.5 µg/L or greater strongly considered the presence of bacterial infection ((30, 31).
Statistical analysis
Continuous variables were expressed as mean ± SD if they were normally distributed and categorical variables were expressed as frequencies (percentages). Chi-square test, independent sample t-test, and repeated measure ANOVA were used for analysis. P-values less than 0.05 were considered statistically significant. All analyses were done using SPSS statistical software version 21 (IBM, Armonk, NY, USA).
Results
We enrolled 40 patients in the study, 6 had to be excluded because CMS administration was less than 72 h (3 were on hemodialysis, one was transferred outside ICU and 2 expired before the 3rd day of the study). Among 34 remaining patients who completed the study protocol, the dominant medical diagnosis was (VAP) in 30 cases (88.2%) and the remaining 4 had bacteremia (11.8%). There were no statistically significant differences in baseline characteristics between the two groups. (Table 1)
The PCT concentrations were measured in both groups at the baseline, day 3 and 5 after the administration of colistin and differences in total (CMS) exposure, according to resolution of clinical signs and decline in the (PCT) concentrations were compared in the two study groups.
The serum (PCT) level changes were not significantly different between groups A and B of (CMS) dose interval groups during the study period (Table 2).
In a two-way repeated measure (ANOVA) the PCT levels between two groups, for the corresponding days did not decrease significantly(p=0.468) and the reduction in the PCT levels throughout the study time was not statistically significant either (p=0.131). (Figure1).
The ICU LOS in groups A and B were, 31.31±10.8 and 32.06±14.1 (P=0.866) and ICU mortalities in groups A and B were, 6 (35.29%) and 4 (22.2%) (P=0.392), respectively.
Discussion
The increase in the frequency of antimicrobial resistance is strongly determined by the choice of antibiotic agents, pattern of their use, infection prevention strategies and the microorganism characteristics. In the current era of the emergence of extensive and serious antibiotic resistance, we have remained with limited choices of anti-microbial agents for the management and reversal of this potentially fatal situation (32).
Reintroduction of colistin as an option for managing MDR gram-negative infections seemed inevitable (7, 8).However, continuing growth of gram negative bacterial resistance and inherent properties of colistin are two main challenging issues complicating determination of the appropriate dosing and intervals of this agent in different critical care settings. Several reports have demonstrated that even the administration of 9 million units per day of CMS, which is 50% or more over the usual recommended dose by the manufacturer, did not provide adequate serum levels for the concentration/MIC ratios needed to manage many MDR strains (12, 16).
In our study, we used colistin only for the treatment of severe infections resistant to other commonly used potent antibiotics. All patients in our study were infected by multi drug-resistant K. pneumonia, A. baumannii and P.aeruginosa. Based on the results of previous studies, we preferred to administer higher doses (9MIU per day) than the standard dose of colistin.
Pharmacokinetic/pharmacodynamics studies have shown colistin to display concentration-dependent killing against gram negative organisms (33, 34)and high-dose and extended interval dosing regimens(13, 17) might be more efficacious in improving clinical outcome. on the contrary, there is some evidences against the extend interval regimen due to the concerns over emergence of resistance to colistin (35).
Demographic and clinical Characteristics of patients
| P - value | 9 MIU Once Daily | 3 MIU TDS | |
|---|---|---|---|
| Age | 0.082 | 45.59 ± 18.3 | 55.71 ± 14.3 |
| APACHEII | 0.765 | 20.47 ± 5.1 | 20.47 ± 5.1 |
| Length of stay | 0.866 | 31.31 ± 10.8 | 32.06 ± 14.1 |
| CMS duration | 0.688 | 12.82 ± 4.3 | 13.53 ± 5.8 |
| Sex (m/f) | 0.999 | (11/6) | (11/6) |
| Source of infection | |||
| BSI, n (%) of cases | 0.57 | 2(11.1%) | 3(17.6%) |
| VAP, n (%) of cases | 15(88.23) | 14(82.4%) | |
| Combination with other antibiotics | |||
| Aminoglycosides | 6 | 3 | |
| B-lactams | 2 | 3 | |
| Quinolones | 3 | 3 | |
PCT concentration and CMS duration
| CMS groups | |||
|---|---|---|---|
| P-value | 9 MIU Once Daily | 3 MIU TDS | Parameters |
| PCT | |||
| 0.602 | 2.34 ± 3.9 | 5.89 ± 16.6 | Baseline |
| 0.056 | 1.24 ± 1.6 | 1.24 ± 2.6 | Day 3 |
| 0.839 | 0.95 ± 2.03 | 0.80 ± 0.9 | Day 5 |
| 0.369 | 14(10) | 14 (4) | CMS duration* |
| 0.866 | 31.31 ± 10.8 | 32.06 ± 14.1 | ICU LOSǂ |
| 0.392 | 6 (35.29%) | 4 (22.22%) | ICU mortality, n (%) |
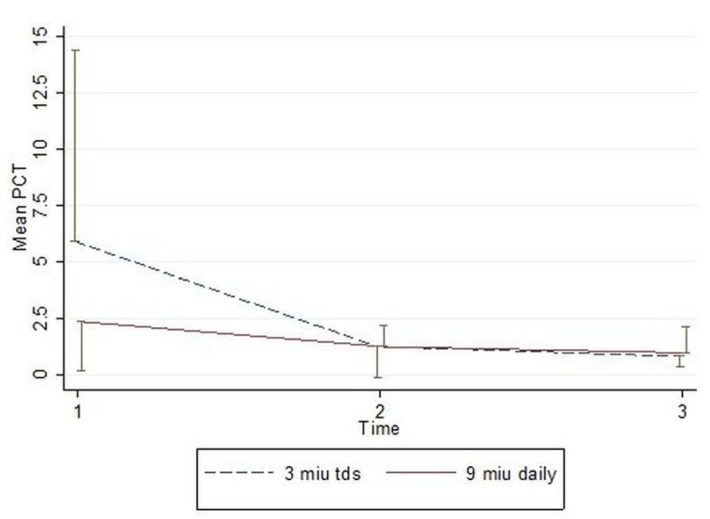
In our study, (CMS) treatment was continued until the surveillance cultures detected that no organisms or clinical symptoms of patients were improved. We attempted to compare the effects of CMS administration in the extended interval of 9 MIU daily infusion (A) vs. 3 MIU every 8 h (B), on the serum PCT reduction trends, ICU LOS, and ICU mortality.
Although the baseline serum (PCT) values in group B (5.89µg/L) were higher than the values in group A (2.34 µg/L) and the rates of (PCT) reduction appeared to be numerically higher in the former group, neither of these values reached statistical significance. Trends in the ICU (LOS) were similar in both groups and although ICU mortality was higher, 6 vs. 4 in groups A compared to group B, these differences were not significant either.
The limitations of our study were single center design and small sample size.
Larger and multicenter studies are required to further elucidate the biomarkers role in the optimization of colistin dosing regimens in order to improve clinical course of antibiotic therapy and to prevent the emergence of colistin resistance.
References
-
1.
Group EEJW. The bacterial challenge: time to react A call to narrow the gap between multidrug-resistant bacteria in the EU and development of new antibacterial agents. Stockholm: European Centre for Disease Prevention and Control; 2009.
-
2.
Sistanizad M, Kouchek M, Miri M, Goharani R, Solouki M, Ayazkhoo L, Foroumand M, Mokhtari M. Carbapenem Restriction and its Effect on Bacterial Resistance in an Intensive Care unit of a Teaching Hospital. Iran. J. Pharm. Res. 2013;12:503-9. [PubMed ID: 24250656].
-
3.
Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 2002;34:634-40.
-
4.
Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006;42:657-68. [PubMed ID: 16447111].
-
5.
Rahal JJ. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect Dis. 2006;43:S95-S9. [PubMed ID: 16894522].
-
6.
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. int. J. Antimicrob. Agents. 2005;25:11-25. [PubMed ID: 15620821].
-
7.
Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005;40:1333-41. [PubMed ID: 15825037].
-
8.
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet. Infect. Dis. 2006;6:589-601. [PubMed ID: 16931410].
-
9.
Michalopoulos A, Tsiodras S, Rellos K, Mentzelopoulos S, Falagas M. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic Clin. Microbiol. Infect. 2005;11:115-21.
-
10.
Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Critical Care. 2003;7:R78. [PubMed ID: 12974973].
-
11.
Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006;50:1953-8. [PubMed ID: 16723551].
-
12.
Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin. Ther. 2008;30:143-51. [PubMed ID: 18343250].
-
13.
Daikos G, Skiada A, Pavleas J, Vafiadi C, Salatas K, Tofas P, Tzanetou K, Markogiannakis A, Thomopoulos G, Vafiadi I. Serum bactericidal activity of three different dosing regimens of colistin with implications for optimum clinical use. J. Chemother. 2010;22:175-8. [PubMed ID: 20566422].
-
14.
Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after IV colistin methanesulfonate administration. CHEST J. 2010;138:1333-9.
-
15.
Garonzik S, Li J, Thamlikitkul V, Paterson D, Shoham S, Jacob J, Silveira F, Forrest A, Nation R. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011;55:3284-94. [PubMed ID: 21555763].
-
16.
Plachouras D, Karvanen M, Friberg L, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009;53:3430-6. [PubMed ID: 19433570].
-
17.
Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin. Infect. Dis. 2012;54:1720-6. [PubMed ID: 22423120].
-
18.
Becker K, Nylen E, White J, Muller B, Snider Jr R. Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J. Clin. Endocrinol. Metab. 2004;89:1512-25. [PubMed ID: 15070906].
-
19.
Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 2004;39:206-17. [PubMed ID: 15307030].
-
20.
Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin. Infect. Dis. 2011;53:379-87. [PubMed ID: 21810753].
-
21.
Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: A systematic review and an economic evaluation. Crit. Care. Med. 2011;39:1792-9. [PubMed ID: 21358400].
-
22.
Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: A systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 2010;38:2229-41. [PubMed ID: 20729729].
-
23.
Soni NJ, Samson DJ, Galaydick JL, Vats V, Huang ES, Aronson N, Pitrak DL. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J. Hosp. Med. 2013;8:530-40. [PubMed ID: 23955852].
-
24.
Matthaiou DK, Ntani G, Kontogiorgi M, Poulakou G, Armaganidis A, Dimopoulos G. An ESICM systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care. Med. 2012;38:940-9. [PubMed ID: 22538461].
-
25.
Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, Zimmerli W, Harbarth S, Tamm M, Muller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am. J. Respir. Crit. Care Med. 2006;174:84-93. [PubMed ID: 16603606].
-
26.
Stolz D, Christ-Crain M, Bingisser R, Leuppi Jr, Miedinger D, Muller C, Huber P, Muller B, Tamm M. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. CHEST J. 2007;131:9-19.
-
27.
Society AT, America IDSo. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J .Respir. Crit. Care. Med. 2005;171:388-416. [PubMed ID: 15699079].
-
28.
Von Lilienfeld-Toal M, Lehmann LE, Raadts AD, Hahn-Ast C, Orlopp KS, Marklein G, Purr I, Cook G, Hoeft A, Glasmacher A. Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia. J. Clin. Microbiol. Infect. 2009;47:2405-10.
-
29.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268-81. [PubMed ID: 21793988].
-
30.
Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000;28:977-83. [PubMed ID: 10809269].
-
31.
Nylen E, Muller B, Becker KL, Snider R. The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin. Infect. Dis. 2003;36:823-4. [PubMed ID: 12627371].
-
32.
Harbarth S. Einfluss des Antibiotikaverbrauchs auf Resistenzbildung und-selektion. AINS-Anästhesiologie· Intensivmedizin· Notfallmedizin· Schmerztherapie. 2007;42:130-5.
-
33.
Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, DʹHondt RE, Jusko WJ, Forrest A, Tsuji BT. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob. Agents Chemother. 2010;54:2051-62. [PubMed ID: 20211900].
-
34.
Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 2007;59:473-7. [PubMed ID: 17289768].
-
35.
Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice-and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 2008;61:636-42. [PubMed ID: 18227094].