Abstract
Keywords
Introduction
Despite the progress in treatment of infectious diseases, infections remain among the most common and costly causes of death and disease around the world, especially in developing countries. The early diagnosis of infection from sterile inflammation is one of the most common problems in nuclear medicine and for this reason several radiopharmaceuticals have been developed to resolve the solution for this difficult situation. However, there are many limitations in developing specific radiopharmaceuticals for clear distinction between infection and inflammation.
67Ga-Citrate, being the most basic radiotracer for this purpose, has a high sensitivity for acute and chronic infections and sterile inflammation (1). There are drawbacks ascribe to this radiopharmaceutical, including its long physical half-life and high-energy radiation. A number of other radiopharmaceuticals labeled with different radioisotopes have been already used, but an ideal agent has not yet been found. Polyclonal and monoclonal immunoglobulins (2, 3) and liposomes labeled with 99mTc (4-6) are nonspecific tracers and may cause certain difficulties. Antibody fragments and antigranulocyte antibodies, chemotactic peptides, cytokines and interleukins also have certain limitations (7). Autologous leukocytes labeled with 111In are still considered by many to be the gold standard for localization of infections but the product must be prepared in a sterile environment. Although leukocytes labeled with 99mTc have the advantages of a low radiation to the patient and an ideal energy, this agent is less stable than 111In-labeled leukocytes (8). Antimicrobial peptides, produced by phagocytes, epithelial cells, endothelial cells, and many other cell types, are an important component of natural immunity against infection (9). In several previous studies, the 99mTc labeled cationic antimicrobial peptide derived from human ubiquicidine (UBI) was introduced for detection of bacterial and fungal infections (10-12).
Broad spectrums of antibiotic agents have been suggested as promising diagnostic tools for the detection of infected lesions. The antibiotic molecules accumulate at the site of infection due to their metabolization by microorganisms (13). The majority of the various antibiotics studied in this regard are those of the quinolones family, second and third generation cephalosporins (14-17). Ciprofloxacin as a quinolone antibiotic labeled with 99mTc has shown a high sensitivity and specificity for infection imaging (18). This antibiotic binds to the DNA gyrase enzyme of bacteria and has shown good accuracy in hip prosthesis infections (19). However, antibiotics encounter the problem of antibiotic resistant bacteria, which is attributed to ciprofloxacin as well (20-23).
Gemifloxacin is an oral broad spectrum quinolone antibacterial agent used in the treatment of acute bacterial aggravation of chronic bronchitis and mild to moderate pneumonia. Gemifloxacin has been shown to be active against most strains of the aerobic gram positive, aerobic gram negative and some other microorganisms (24). In order to use the increased ability of the gemifloxacin for localization of infection in the present study, radiolabeling of the gemifloxacin with 99mTc via carbonyl core was evaluated. Optimization of labeling condition, stability in human serum, lipophilicity, binding with Staphylococcus aureus and biodistribution in infected mice for the labeled compound were studied.
Experimental
General
Gemifloxacin mesylate obtained from Life Sciences Pharmaceuticals (LSP). All other chemicals obtained from Sigma-Aldrich and were used without further purification. Technetium-99m as sodium pertechnetate (Na99mTcO4) was obtained from an in-house 99Mo/99mTc generator using 0.9% saline. All reactions were performed and monitored using an analytical reverse-phase high performance liquid chromatography (RP-HPLC) on a JASCO 880-PU intelligent pump HPLC system (Tokyo, Japan) equipped with a multiwavelength detector and a flow-through Raytest-Gabi γ-detector. CC 250/4.6 Nucleosil 120-5 C-18 column from Teknokroma was used for HPLC. 0.1% trifluoroacetic acid/water (Solvent A) and 0.1% trifluoroacetic acid/acetonitrile (Solvent B) were used as a mobile phase in the following gradient: 0 min 95% A (5% B), 5 min 95% A (5% B), 25 min 0% A (100% B), 30 min 0% A (100% B), flow = 1 mL/min, λ = 280 nm. Radioactivity measurements were carried out using Na (Tl) scintillation counter (ORTEC Model 4001 M Minibin & Power Supply).
Tricarbonyl
The precursor [99mTc (CO)3(H2O)3]+ was prepared according to the reported procedure by Mirshojaei et al [16]. Briefly, in a closed vial 4.5 mg Na2CO3, 5.5 mg NaBH4 and 20 mg sodium potassium tartarate were added and the vial was flushed with CO and after that the 1100 MBq activity that was eluted from a commercial 99Mo/99mTc generator was added and heated to 95 °C for 30 min. After cooling the vial to room temperature, using 1 N HCl the solution was neutralized to pH = 7.
Radiochemical analysis
Radiochemical purity of the precursor was checked by TLC (silica gel 60 Merck) using 99.9% methanol, 0.1% concentrated HCl as a solvent. The tricarbonyl precursors reveal a retention factor (Rf) of 0.3, and unreacted 99mTcO4- show a Rf of 0.7 while Colloidal TcO2 remain at the origin. The radioactivity was quantified by cutting the strip (1.5 × 10 cm2) into 1 cm pieces and counting in a well type gamma counter.
Preparation of 99mTc(CO)3-gemifloxacin
A stock solution of gemifloxacin (concentration 20 mg/mL) was prepared by dissolving gemifloxacin in water; from this stock solution different amounts of gemifloxacin (0.5-5 mg) were carefully transferred to a vial. To this solution [99mTc (CO)3(H2O)3]+ precursor (100 µL, 370 MBq) was added and while various range of pH (1-9) was adjusted, its labeling was completed by incubation of vial in a boiling water bath for 30 min. After cooling down to room temperature (15 min) the formulation was checked for preparation of complex.
In-vitro Stability
Stability of 99mTc(CO)3-gemifloxacin was evaluated in NaCl 0.9% (W/V) and in human serum. Aliquots were taken out at 1, 4, 6, 12 and 24 h post reconstitution at room temperature and analyzed by HPLC. A 100 μL of labeled formulation was added to 1 mL of freshly prepared human serum, and the mixture was incubated in a 37 °C environment. Also 100 μL aliquots were removed at the same time points and treated with 100 μL of alcohol. Samples were centrifuged for 5 min at 3000 rpm to precipitate serum proteins and for supernatants, chromatography was performed.
Log P values
For partition coefficient, 0.5 mL of the 99mTc labeled formulation was mixed with 0.5 mL of octanol in a 2 mL micro tube. The tube was vigorously vortexed over a period of 10 min and centrifuged at 3000 g for 5 min. Three aliquots of 100 μL were sampled from each layer and counted in the γ counter. The averaged activities from the aqueous and the octanol layers were used to calculate the log P values. The octanol-to-water partition coefficient (Po/w) was calculated by dividing the counts of the octanol phase by that of the aqueous phase.
Binding to bacteria
100 µL of the 99mTc(CO)3-gemifloxacin was transferred to a test tube. Then, 0.9 mL of 50% (v/v) 0.01 M acetic acid in phosphate buffer (pH = 7.5) containing approximately 1×108 colony forming units (CFU) per mL viable S. aureus was added. The mixture was incubated for 1 h at 4 ºC and thereafter the vials were centrifuged in a pre-cooled centrifuge for 5 min at 2000 g at 4 ºC. The supernatant was removed, and analyzed by HPLC method for evaluation of stability of 99mTc labeled compound in incubated medium and binding condition. The bacterial pellet was gently re-suspended in 1 mL of buffer and re-centrifuged as above. The supernatant was removed and the radioactivity in the bacterial pellet was measured by a gamma counter for percent of bounded activity.
Biodistribution in infected mice
Animal experiments were performed in compliance with the regulations of our institution and with generally accepted guidelines governing such work. Male Balb/c mice, weighing 25-30 g were infected by injection 0.1 mL of saline containing 1×108 CFU S. aureus bacteria into right tight muscle. After 24 h, a group of three balb/c mice received 3.7 MBq of 99mTc(CO)3-gemifloxacin in volume of 0.1 ml via a tail vein. The mice were sacrificed at different post injection times (1, 4 and 24 h) and the tissues and organs of interest were collected, immediately weighed and counted in a NaI well-type γ-counter. The uptake was expressed as the percentage of the injected dose per gram tissue (%ID/g tissue). The values are expressed as mean ± SD.
Scintigraphy studies
At different time point after injection, accumulation of the tracer in infected area was assessed by planar scintigraphy using the single head gamma camera (small area mobile, Siemens, 140 KeV high sensitivity parallel whole collimator and 10% window around 140 KeV). Before the imaging mice were anesthetized with 0.05 mL ketamine 10% (3.3 mg) and 0.05 mL xylazine 2% (1.33 mg) intra-peritoneally. After about 5 min, the animal was fixed on a board by covering with pieces of cloth for immobilization during the scanning.
Results and Discussion
The significant property of the precursor [99mTc (CO)3(H2O)3]+ provided by Alberto et al. (25) leads to further interest to the development of new 99mTc-based radioconjugates. The technetium tricarbonyl core is able to forms highly stable complexes with a wide range of ligand systems containing donor atom such as nitrogen, oxygen and sulfur (26, 27). Previous study in structural characterization of rhenium(I) tricarbonyl complexes with the bidentate ligands showed that bidentate ligands react readily with the precursor to give complexes of the type [Re(CO)3(LL)X] (where LL = bidentate ligand) with square bipyramidal geometry which may be further elaborated for potential radiopharmaceutical applications (28). Due to presence of electron donor atoms such as oxygen in gemifloxacin structure, the tricarbonyl precursor can easily react with it and with replacement of its water component a complex is formed (Figure 1). Although the exact complex structure is not known, the proposed structure of the bidentate radiocomplex will have square bipyramidal geometry (28). Further researches seem to be required to determine the exact structure of this new conjugate.
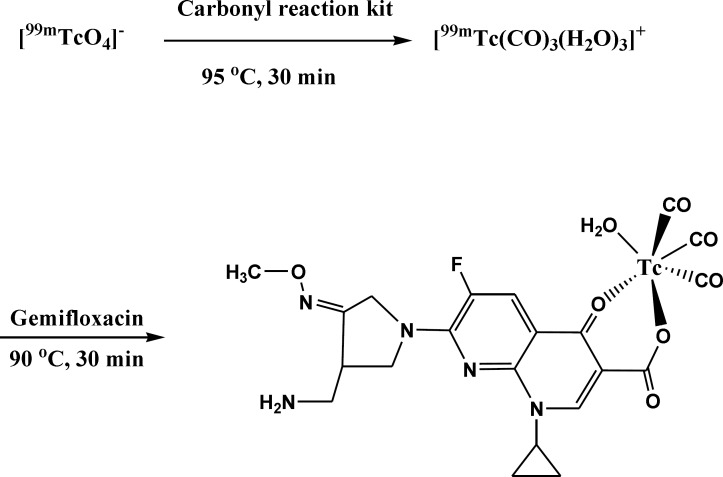
Influence of different amount of gemifloxacin ligand on carbonyl labeling efficiency. Values are expressed as mean ± standard deviation (n = 3
| Gemifloxacin (mg) | Labeling yield (%) |
|---|---|
| 0.5 | 3 ± 4.1 |
| 1.0 | 75.3 ± 3.6 |
| 1.5 | 90.4 ± 2.3 |
| 2.0 | 98.2 ± 1.1 |
| 2.5 | 97.8 ± 1.4 |
| 5.0 | 95.1 ± 2.1 |
Biodistribution of 99mTc(CO)3-Gemifloxacin in mice (%ID/g ± SD, n = 3
| Post-injection time (h) | |||
|---|---|---|---|
| 1 | 4 | 24 | |
| Blood | 12.15 ± 1.60 | 7.47 ± 1.35 | 2.57 ± 0.18 |
| Heart | 3.16 ± 1.12 | 3.23 ± 0.82 | 0.98 ± 0.15 |
| Lung | 8.51 ± 1.35 | 4.86 ± 0.82 | 1.72 ± 0.31 |
| Stomach Bone | 1.41 ± 0.51 | 1.53 ± 0.21 | 8.00 ± 1.39 |
| Intestine | 2.17 ± 0.64 | 2.11 ± 0.36 | 0.60 ± 0.29 |
| Thyroid | 5.74 ± 1.58 | 6.06 ± 0.72 | 1.37 ± 0.38 |
| Liver | 4.06 ± 1.33 | 3.34 ± 0.35 | 1.19 ± 0.21 |
| Spleen | 14.22 ± 1.38 | 13.18 ± 1.94 | 6.27 ± 0.95 |
| Kidney | 2.78 ± 0.35 | 1.83 ± 0.21 | 1.09 ± 0.28 |
| Non-infected muscle | 13.92 ± 0.47 | 8.59 ± 1.21 | 4.36 ± 0.75 |
| S. aureus infected muscle | 1.38 ± 0.21 | 0.89 ± 0.10 | 0.37 ± 0.04 |
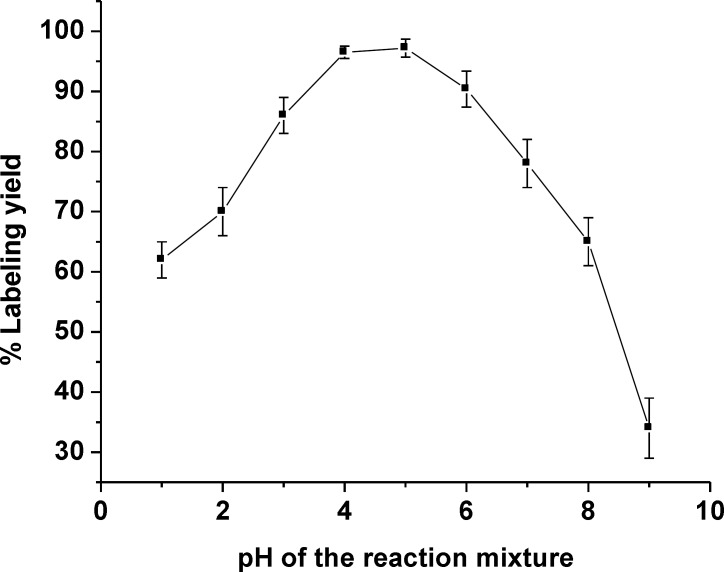
Optimization of the radiolabeling condition was performed by varying reaction parameters such as the amount of ligand gemifloxacin and the pH value of reaction system, to identify an easy-to-use synthetic route giving a high radiolabeling yield of the product. Reducing the amount of ligand used in the formulation is still highly desirable. Therefore, one of the objectives was to reduce the amount of ligand gemifloxacin used in the labeling process. The amount of 2 mg gemifloxacin was the sufficient amount of ligand in formulation to reach a high labeling yield complex formation (Table 1). The effect of reaction pH was also investigated and the optimal pH range to produce a high labeling yield of 99mTc(CO)3-gemifloxacin was found to be between pH = 4-5 and goes down to low labeling yield at pH out of this range (Figure 2).
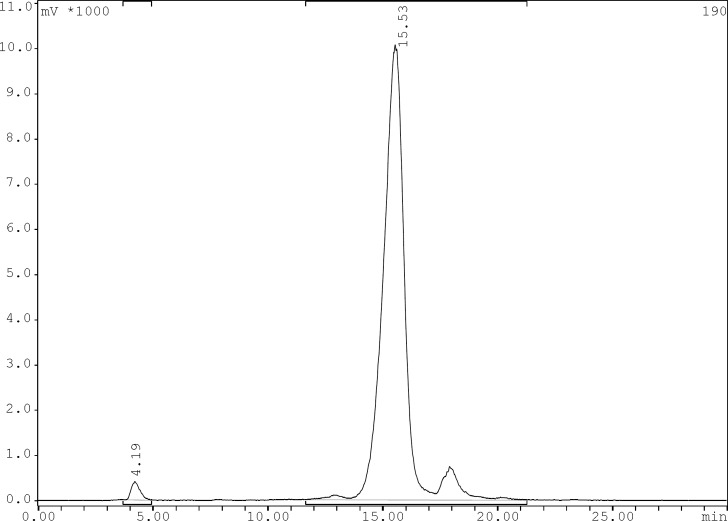
Radiochemical purity of the 99mTc-tricarbonyl core and 99mTc-tricarbonyl-ligand were determined by TLC and reversed phase HPLC using the gradient systems consisted of 0.1% trifluoroacetic acid/water (Solvent A) and 0.1% trifluoroacetic acid/acetonitrile (Solvent B). The preparation yield for [99mTc (CO)3(H2O)3]+ was > 95% acquired via TLC. In TLC the tricarbonyl precursors reveal a Rf of 0.3, and less than 3% of total activity was moved and counted in Rf of 0.7 belonged to 99mTcO4- and only minimal activity (less than 1%) remained in origin corresponding to 99mTc-colloid. The 99mTc-tricarbonyl-gemifloxacin was characterized by HPLC which prepared in > 95% yield at specific activity of 0.14 GBq/μmol. The HPLC retention times of 99mTcO4- was 4.19 min and for 99mTc(CO)3-gemifloxacin was 15.53 min (Figure 3).
The radiochemical purity of the 99mTc-tricarbonyl-gemifloxacin was > 95% over the observed period of 24 h. No decomposition of the complex was observed in this time period, suggesting its high stability in normal saline at room temperature. Radiochemical stability of labeled antibiotic in human serum was 84 ± 2.4% after 24 h. So far the main drawback in labeling of ciprofloxacin and its similar structures are colloid impurity and instability which were the subjects of discussion in previous studies by different groups (16, 29-31) but here with design of a new labeling method by tricarbonyl core and optimization of labeling condition high labeling yield and stability for 99mTc(CO)3-gemifloxacin is achieved.
The partition coefficient of the radiolabeled complex was determined by distribution in octanol and water, and the lipophilicity (log P) of 99mTc(CO)3-gemifloxacin was found to be 0.7 ± 0.09, signifying its lipophilicity which could explain the accumulation of the radioligand in the liver. In-vitro binding of 99mTc-tricarbonyl-gemifloxacin to S. aureus showed that 55 % of radioactivity bound to bacteria while no decomposition of 99mTc labeled compound was observed in HPLC in evaluated condition.
Biological evaluation of 99mTc(CO)3-gemifloxacin complex was performed in Balb/c mice. The results are shown in Table 2. Clearance from the blood circulation was mild with 7.47 ± 1.35 %ID/g remaining in the blood at 4 h. Moderate clearance from the kidney (13.92 ± 0.47 %ID/g at 1 h and 8.59 ± 1.21 %ID/g at 4 h post injection) followed by slow liver clearance were observed (14.22 ± 1.38 %ID/g at 1 h and 13.18 ± 1.94 %ID/g at 4 h post injection). The presence of high activity in liver and kidney suggesting that the hepatobiliary and urinary systems are the major routes of excretion of the administered radioactivity.
The radioactivity concentration of infected muscle by S. aureus at 1 h post injection was 2.93 ± 0.30 %ID/g which decreased to 1.74 ± 0.01 %ID/g at 4 h post injection. The ratio of activity compared to non infected muscle was more than two fold (T/NT = 2.12 ± 0.24 at 1 h post injection) which was more than ratios reported previously for 99mTc(CO)3-ofloxacin (1.63 ± 0.11 at 1 h post injection) (17). Results show that more than 90% the bounded activity in infected area at 1 h (T/NT = 2.12%) without any variation remains at 4 h period (T/NT = 1.95%) which is maybe due to the clearance of non specific uptake and on the other hand this high retention shows 99mTc(CO)3-gemifloxacin have specific affinity to bacterial infection site, although the T/NT ratio at 4 h post injection is lower than 1 h post injection.
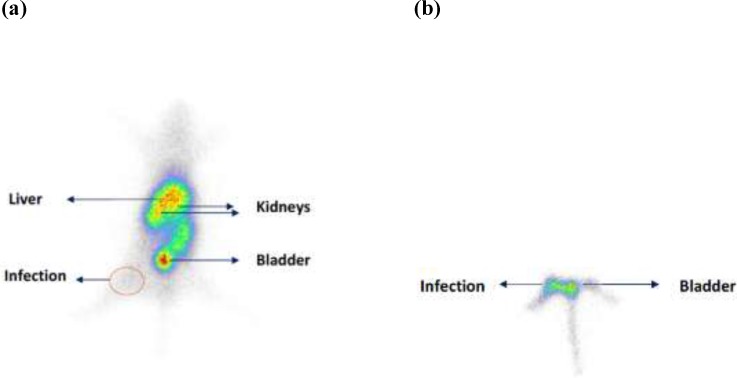
Scintigraphic study at 1 h post injection showed uptake for radioligand in infections site (Figure 4). The uptake in all organs was decreased significantly after 24 h which shows that elimination is time depended and the early time up to 1 h is the best time for infection detection.
Conclusion
In this study, we have shown preparation and evaluation of an infection imaging agent 99mTc(CO)3-gemifloxacin with high labeling yield. Based on the data obtained from this study, the product was stable, reproducible with high labeling efficiency with desirable characteristics making it a promising agent for imaging of infectious lesions. According to the results of in-vivo biodistribution studies, we found that this complex have infection site uptake with a good retention time. These promising characteristics make our new designed labeled conjugate as a very suitable candidate for diagnostic of infection sites in nuclear medicine. Further investigation on the ability to bind and detection of the other bacteria strains specially gram negative bacteria should be pursed.
Acknowledgements
References
-
1.
Palestro CJ. The current role of gallium imaging in infection. Semin. Nucl. Med. 1994;24:128-41. [PubMed ID: 8023169].
-
2.
Calame W, Welling MM, Feitsma RIJ, Ensing GJ, Pauwels EKJ. Improved detection of staphylococcal infection by monomeric and protein A-purified polyclonal human immunoglobulins. Eur. J. Nucl. Med. 1993;20:490-4. [PubMed ID: 8339732].
-
3.
Welling MM, Feitsma RIJ, Calame W, Pauwels EKJ. Localization of a bacterial infection with Tc-99m-labeled human IgG: further improvement with enriched IgG subclass preparation. Nucl. Med. Commun. 1997;18:1057-64. [PubMed ID: 9423206].
-
4.
Dams ETM, Oyen WJG, Boerman OC, Laverman P, Naber TH, Storm G, Corstens FH. Tc-99m-PEG-liposomes for the scintigraphic detection of infection and inflammation: clinical evaluation. J. Nucl. Med. 2000;41:622-30. [PubMed ID: 10768562].
-
5.
Hnatowich D, Virzi F, Rusckowski M. Investigation of avidin and biotin for imaging investigation. J. Nucl. Med. 1987;28:1294-302. [PubMed ID: 3612292].
-
6.
Fischman AJ, Rauh D, Solomon H, Babich JW, Tompkins RG, Kroon D, Strauss HW, Rubin RH. In vivo bioactivity and biodistribution of chemotactic peptide analogs in nonhuman primates. J. Nucl. Med. 1993;34:2130-4. [PubMed ID: 8254400].
-
7.
Peters AM. The utility of Tc-99m-HMPAO leucocytes for imaging infection. Semin. Nucl. Med. 1994;24:110-27. [PubMed ID: 8023168].
-
8.
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389-95. [PubMed ID: 11807545].
-
9.
Welling MM, Nibbering PH, Paulusma-Annema A, Hiemstra PS, Pauwels EKJ, Calame W. Imaging of bacterial infections with 99mTc-labeled human neutrophil peptide-1. J. Nucl. Med. 1999;40:2073-80. [PubMed ID: 10616888].
-
10.
Gandomkar M, Najafi R, Mazidi M, Mirfallah SH, Goudarzi M. Three different procedures in labeling of Ubiquicidin with technetium 99m: a comparative study. Iran. J. Radiat. Res. 2009;7:97-104.
-
11.
Gandomkar M, Najafi R, Mazidi M, Goudarzi M, Mirfallah SH. New peptide based freeze-dried kit [99mTc-HYNIC]-UBI 29-41 as a human specific infection imaging agent. Iran. J. Nucl. Med. 2008;16:25-30.
-
12.
Gandomkar M, Najafi R, Shafiei M, Mazidi M, Goudarzi M, Mirfallah SH, Ebrahimi F, Heydarpor HR, Abdie N. Clinical evaluation of antimicrobial peptide [(99m)Tc/Tricine/HYNIC(0)]ubiquicidin 29-41 as a human-specific infection imaging agent. Nucl. Med. Biol. 2009;36:199-205. [PubMed ID: 19217532].
-
13.
Fournier B, Zhao X, Lu T, Drlica K, Hooper DC. Selective targeting of topoisomerase IV and DNA gyrase in Staphylococcus aureus: different patterns of quinolone-induced inhibition of DNA synthesis. Antimicrob. Agents. Chemother. 2000;44:2160-5. [PubMed ID: 10898691].
-
14.
Mirshojaei SF, Erfani M, Ebrahimi SES, Talebi MH, Haj Hassan Abbasi F. Freeze-dried cold kit for preparation of 99mTc-ciprofloxacin as an infection imaging agent. Iran. J. Nucl. Med. 2010;18:45-51.
-
15.
Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S. Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur. J. Nucl. Med. 1997;24:553-6. [PubMed ID: 9142737].
-
16.
Mirshojaei SF, Gandomkar M, Najafi R, Ebrahimi SES, Babaei MH, Shafiei A, Talebi MH. Radio labeling, quality control and biodistribution of 99mTc-cefotaxime as an infection imaging agent. J. Radioanal. Nucl. Chem. 2011;287:21-5.
-
17.
Erfani M, Doroudi A, Hadisi L, Andishmand A, Mirshojaei SF, Shafiei M. 99mTc-tricabonyl labeling of ofloxacin and its biological evaluation in Staphylococcus aureus as an infection imaging agent. J. Label. Compd. Radiopharm. 2013;56:627-31.
-
18.
Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J. Clinical evaluation of technetium-99m Infecton for the localisation of bacterial infection. Eur. J. Nucl. Med. 1997;24:553-6. [PubMed ID: 9142737].
-
19.
Larikka MJ, Ahonen AK, Niemela O, Junila JA, Hamalainen MM, Britton K, Syrjala HP. Comparison of 99mTc ciprofloxacin, 99mTc white blood cell and threephase bone imaging in the diagnosis of hip prosthesis infections: improved diagnostic accuracy with extended imaging time. Nucl. Med. Commun. 2002;23:655-61. [PubMed ID: 12089488].
-
20.
Limoncu MH, Ermertcan S, Cetin CB, Cosar G, Dinc G. Emergence of phenotypic resistance to ciprofloxacin and levofloxacin in methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains. Int. J. Antimicrob. Agents. 2003;5:420-4.
-
21.
Fewton KA, Ison C, Johnson AP, Rudd E, Soltani M, Martin I, Nichols T, Livermore DM. Ciprofloxacin resistance in Neisseria gonorrhoeae in England and Wales in 2002. Lancet. 2003;361:1867-9. [PubMed ID: 12788575].
-
22.
Jang CH, Park SY. Emergence of ciprofloxacin-resistant pseudomonas in pediatric otitis media. Int. J. Pediatr. Otorhinolaryngol. 2003;4:313-6.
-
23.
de la campa AG, Ferrandiz MJ, Tabau F, Pallares R, Manresa F, Linares J. Genetic characterization of fluoroquinolone-resistant Streptococcus pneumonia strains isolated during ciprofloxacin therapy from a patient with bronchiectasis. Antimicrob. Agents. Chemother. 2003;4:1419-22.
-
24.
Calvo A, Gimenez MJ. Ex vivo serum activity (Killing Rates) after gemifloxacin 320 mg versus trovafloxacin 200 mg single doses against ciprofloxacin-susceptible and-resistant Streptococcus pneumoniae. Int. J. Antimicrob. Agents. 2002;20:144-6. [PubMed ID: 12297365].
-
25.
Alberto R, Schibli R, Egli A, Schubiger PA. A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]- in aqueous solution and its reaction with a bifunctional ligand. J. Am. Chem. Soc. 1998;120:7987-8.
-
26.
Pak JK, Benny P, Spingler B, Ortner K, Alberto R. Nε functionalization of metal and organic protected L-Histidine for a highly efficient, direct labelling of biomolecules with [Tc(OH2)3(CO)3]+. Chem. Eur. J. 2003;9:2053-61. [PubMed ID: 12740853].
-
27.
Lazarova N, Babich J, Valliant J, Schaffer P, James S, Zubieta J. Thiol-and thioether-based bifunctional chelates for the {M(CO)3}+ core (M = Tc, Re). Inorg. Chem. 2005;44:6763-70. [PubMed ID: 16156635].
-
28.
Chen X, Femia FJ, Babich JW, Zubieta J. Synthesis and structural characterization of rhenium(I) tricarbonyl complexes with the bidentate ligands o-diphenylphosphino)benzaldehyde (P∩O) and o-[(diphenylphosphino)benzylidene]analine (P∩N). Inorganica. Chim. Acta. 2001;27:147-52.
-
29.
Solanki KK, Bomanji J, Siraj QH, Small M, Britton KE. Tc-99m Infecton: A new class of radiopharmaceutical for imaging infection. J. Nucl. Med. 1993;34(Suppl):119P.
-
30.
Motaleb MA. Preparation and biodistribution of 99mTc-lomefloxacin and 99mTc-ofloxacin complexes. J. Radioanal. Nucl. Chem. 2007;272:95-9.
-
31.
Bhardwaj N, Bhatnagar A, Singh AK. Development and evaluation of a single vial cold kit for infection imaging: Tc-99m ciprofloxacin. World J. Nucl. Med. 2005;4:244-51.