Abstract
Keywords
Introduction
Hemophilia is an X-linked disorder and rare chromosomal disease that leads to excessive bleeding in men. Reduction of clotting factor VIII concentration causes Hemophilia A (1). The existence of the inhibitor causes severe complications in patients with Hemophilia A (2). To treat patients with Hemophilia resistant against factor VIII, recombinant activated factor Vll (rFVIIa) or activated prothrombin complex concentrates (aPCC) are usually used as bypassing agents (3). AryoSeven® is a biogeneric of rFVIIa marketed in Iran which has recently shown a comparable efficacy with that of NovoSeven (4) and is the available rFVIIa product used to manage bleeding in Hemophilia patients with inhibitors in Iran AryoSevenTM [package insert]. On the other hand, FEIBA® is the sole available aPCC in Iran. Administration of bypassing agents in patients with high titre inhibitors for bleeding episodes is very costly (5). Therefore, economic analysis of available bypassing agents could provide useful data to select eligible alternative therapeutic strategies to treat patients with Haemophilia (6, 7). Although most of the studies show that bypassing agents are effective, none of them is permanently effective (8). The current study aimed to evaluate the cost-effectiveness of bypassing agents to treat patients with Haemophilia and high titre inhibitors through decision analytic model, according to the Iranian health care system.
Experimental
Methods
Considering the nature of the disease and small size of the study population, an open-label, non-randomized, cross-over and multi-center study was designed and performed in five Hemophilia centers in Iran. The Hemophilia A patients with high titer inhibitors (≥ 5 Bethesda Units) were selected for the study.Each patient was treated and evaluated for two separate bleeding events: one with AryoSeven based protocol (protocol A) and the other one with FEIBA based protocol (protocol F). As part of the study to detect and evaluate the influence of tendency of the patients toward different types of bypassing agents, patients were categorized into three groups: group A (patients tending to use AryoSeven), group F (patients tending to use FEIBA), and group O (patients indifferent to receive either product). Both protocols A and F were evaluated in all of the patients. Given that the current study was done based on the study by FENOC (2); hence, the subjects were selected in accordance with the inclusion criteria for the patients in his study. Patients with other clotting problems, advanced liver problems for short life expectancy, those who received blood products within five days prior to the test, and the patients with joint bleeding in the joint under study within seven days prior to their admission for the treatment were excluded.
Study procedures
All subjects signed the informed consent letter prior to enrolling the study. After categorization the patients were evaluated according to two bleeding events. According to the scoring scale of the patients in the study by Kavakli et al. (9), the therapeutic protocol was employed in the first two hours after the onset of the pain and the subjects` clinical symptoms and the responses were recorded after 1, 3, 6, 9, 12, 18, and 24 h of the product infusion. Protocols A and F (below section) were respectively employed in all groups after a bleeding event. Case Report Form (CRF) was completed by the specialists to compare efficacy of the procedures in each bleeding event. Study duration was from enrolment to completion of both bleeding events.
Therapeutic protocols
According to the injection protocol of the two products, in protocol A (treatment with AryoSeven), all subjects received 90-120 μg/kg body weight on arrival (time 0); 100 μg/kg was considered as the mean dosage (2). Based on the subjective responses a specific therapeutic decision was made every three hours. In case of two failures with 90 μg/kg, a dose of 270 μg/kg was used. If no improvement was observed three hours after the next 270 μg/kg dosage, it was considered as treatment failure for non-responders. In protocol F (treatment with FEIBA), all subjects received 50-75 U/kg body weight on arrival (time 0). Next dosage (75-100 U/kg) was injected after nine hours only if no relieving sign was observed. When there was no response, it was considered treatment failure.
Effectiveness of Therapy in Patients
A scoring system was used to determine the effectiveness of the applied therapies (Table 1). Similar scoring systems had been employed in other studies for clinical assessment of efficacy (9). Two outcomes of pain and the extent of the movement limitation were evaluated in all subjects. If pain and/or movement limitation improved significantly, the score “++” was recorded. But if they were worse or even not better, the score was recorded as “-“, except for hours 1 and 3 of assessment when score”+” was recorded if no changes were observed in the symptoms of the subject. Total + scores after 24 h were accounted as efficacy score of the treatment.
Evaluation of the Patients` Clinical Condition
| Outcome | Pain | Limitation in Range of Motion (ROM) | ||||
|---|---|---|---|---|---|---|
| Worse | No difference | Better | Worse | No difference | Better | |
| 1 | - | + | ++ | - | + | ++ |
| 3 | - | + | ++ | - | + | ++ |
| 6 | - | - | ++ | - | - | ++ |
| 9 | - | - | ++ | - | - | ++ |
| 12 | - | - | ++ | - | - | ++ |
| 18 | - | - | ++ | - | - | ++ |
| 24 | - | - | ++ | - | - | ++ |
Therapy Success and Therapy Failure
Scores ≥ 16 + out of 28 were considered as therapy success. Scores less than 16 + and/or clinical necessity to switch or add another bypassing agent was considered as therapy failure.
Determination of Treatment Cost
To collect data concerning cost, a table was developed based on the previous studies conducted in Iran or other countries. All direct medical and non-medical costs were collected. Hence, direct non-medical costs such as transportation cost were included. Therefor indirect and intangible costs were ignored (Table 2).
Components of Direct Medical Costs and Direct Non-Medical Costs.
| Type | Components | |
|---|---|---|
| Direct Medical Costs | Drug Costs | Bypassing agents drug costs, other drug costs |
| Out-Patients Costs | Imaging costs , CT scan costs, blood test costs, bone scan costs,… | |
| In-Patients Costs | Hospitalization costs | |
| Direct Non-Medical Costs | Nursing Costs | Nursing home care Costs |
| Adaptation Costs | Costs of compatibility of facilities at home and workplace | |
| Traveling Costs | Traveling cost for receiving of care |
Ethical Considerations
The Ethical Committee of Shahid Beheshti University of Medical Sciences approved the current study, based on Helsinki declaration. The present clinical trial was registered in IRCT website, under ID No.2013020612380N1.
DecisionAnalyticModel
To provide an optimized strategy to treat Hemophilia patients with inhibitors, a decision analytic model was designed. The two mentioned strategies used FEIBA vs. AryoSeven on the first line of treatment. Data regarding the effectiveness of therapeutic protocols and also the related costs were transferred to the decision tree model. The Tree Age Pro 2011™ software was used in the study for modelling (10). To calculate the cost of medicines, basic prices extracted from the Iranian Food and Drug Organization (FDO) were used, since it is an official drug pricing reference in Iran. One-way and two-way sensitivity analyses were used to assess the robustness of the results. A frequency domain for sensitivity analysis was 25% increase/decrease of the baseline results. According to the Central Bank of Iran the official exchange rate was US$1 = 25,430 Rls. (2014), used to convert costs to the USD.
Results
16 Out of 36 subjects were excluded due to refusing to stay in the protocol and lack of cooperation and protocol deviation during the treatment. Hence, clinical and cost data on 20 patients with 40 bleeding episodes were extracted. All patients were male with the mean age of 17.2 years. Effectiveness scores are shown in Table 3, based on the type of the received protocol in each time point of assessment.
Effectiveness Scores of Patients with Haemophilia A for FEIBA and AryoSeven
| Patients | Mean * | Mean* |
|---|---|---|
| 1 | 2 (50%) | 2.12 (53%) |
| 3 | 2.2 (55%) | 2.64 (66%) |
| 6 | 1.6 (40%) | 2.4 (60%) |
| 9 | 2.4 (60%) | 2.12 (53%) |
| 12 | 3.08 (77%) | 3.6 (90%) |
| 18 | 3.4 (85%) | 3.8 (95%) |
| 24 | 3.6 (90%) | 3.8 (95%) |
Statistical Relationship between the Variables
To statistically analyze the therapy success correlation, the mean of dosage, number of patients with treatment success and also effectiveness scores were used based on the applied therapeutic protocol. To assess the normality of data the Kolmogorov-Smirnov and Shapiro-Wilk tests were used. Based on normal and non-normal data, appropriate statistical tests were employed. Statistical parametric and non-parametric tests were used when the distributions were normal and non-normal, respectively. To determine the relationship between treatment success of protocol A in the groups, Chi-square and the Fisher Exact tests were used. The obtained results showed no statistically significant differences between the groups receiving protocol A (P = 0.53), however group F had statistically significant success with protocol F, compared to the other groups (P = 0.03). Besides, the number of successes with each protocol was statistically equal in all of the groups (Table 4). Although mean dosage of product in protocol A seems to be more in group F and O compared with group A, there were no statistically significant differences between protocols A and F in the treatment groups (P-Value in protocols A and F were 0.18 and 0.19 respectively Table 4. Due to abnormality of data, the statistical relationship between the effectiveness scores of protocol A was evaluated by non-parametric Kruskal-Wallis test (P = 0.31). Mean effectiveness score with protocol F was observed in group F (P = 0.01) but there was no difference between the effectiveness of the protocols in groups A and O (Table 5). No statistically significant difference was observed between the effectiveness scores of the subjects receiving protocols A and F (Table 5(.Several tests showed no statistically significant difference between the carry-over effect on the variables.
Treatment Success and Mean Dosage of Products with Each Protocol in the Treatment Groups
| Statistical Analysis | Treatment Groups | Description of Parameter | |||||
|---|---|---|---|---|---|---|---|
| Group O | Group F | Group A | |||||
| Failure | Success | Failure | Success | *Failure | Success | ||
| 0.53 | 2 | 2 | 2 | 7 | 1 | 6 | The number of patients in protocol A |
| 0.18 | 444 | 529 | 274 | The mean dosage of Aryoseven used at every event | |||
| 0.03 | -- | 4 | -- | 9 | 3 | 4 | The number of patients in protocol F |
| 0.19 | 121 | 79 | 94 | The mean dosage of FEIBA used at every event | |||
| 0.42 | 0.47 | 0.55 | Statistical Analysis on number of success in each group (P value) | ||||
Effectiveness Score of Protocols in Different Groups of Patients
| Mean of effectiveness Score | |||||
|---|---|---|---|---|---|
| All | Group O | Group F | Group A | Treatment protocol | |
| 18 | 16 | 17 | 21 | Protocol A | |
| 20 | 20 | 25 | 15 | Protocol F | |
| 0.43 | 0.68 | 0.01 | 0.12 | P-Value | |
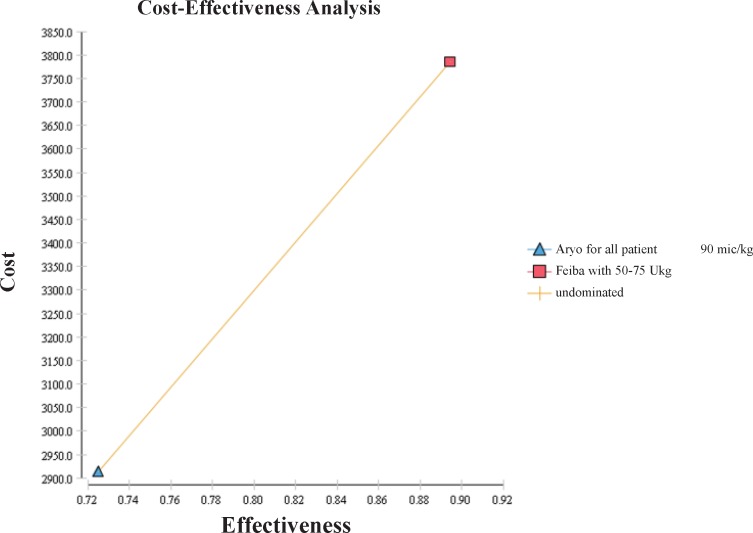
Cost-Effectiveness Analysis
Results of the cost-effectiveness analysis showed that administration of 90 µg kg-1 AryoSeven as the first line treatment with the cost of US $ 2,912 and 72% therapy success in one bleeding episode was more cost-effective compared to administration of 50-75 µg kg-1 FEIBA as the first line of treatment with the cost of US$ 3,785 and 89% therapy success (Table 6 and Figure 1). The incremental cost-effectiveness ratio analysis (ICER) was US$ 5,146 to manage an episode of bleeding to get one more unit of effectiveness using FEIBA vs. AryoSeven as the first line of treatment. Although the results of the study confirmed cost effectiveness of AryoSeven compared to FEIBA, it should be mentioned that according to Table 6 these strategies are undominated.
Cost-Effectiveness Rankings between the two Strategies
| ICER | Incremental Effectiveness | Effectiveness | Incremental Cost | cost | strategy | Rank all | |
|---|---|---|---|---|---|---|---|
| --- | --- | 72% | -- | US$2912 | AryoSeven for all patients with 90-120 µg kg-1 | 1 | Undominated |
| US$5146 | 17% | 89% | US$873 | US$3785 | FEIBA for all patients with 50-75 U kg-1 | 2 | |
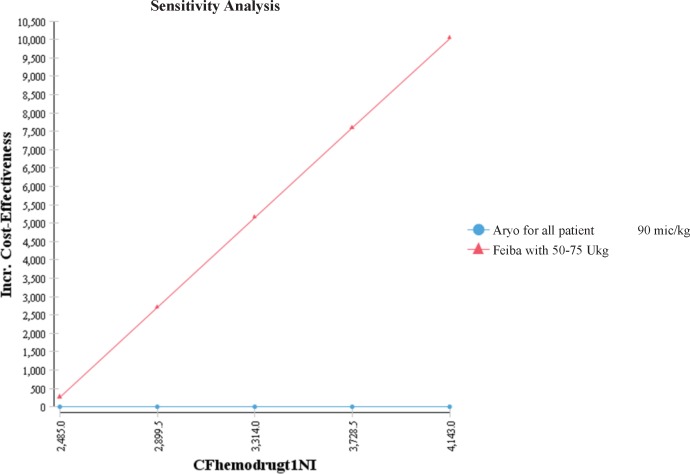
Sensitivity Analysis
To evaluate the results of sensitivity, one-way and two-way sensitivity analyses were employed. Results showed that the level of ICER was sensitive to AryoSeven cost changes in different hours, with more sensitivity at the early hours of treatment. Also, a one-way sensitivity analysis indicated the sensitivity of ICER to the changes in FEIBA cost, which was in maximum and minimum in the first and ninth hours of treatment, respectively (Figure 2).
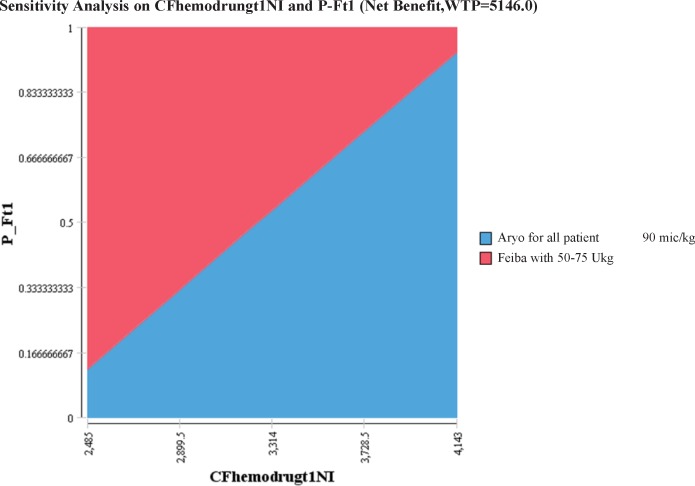
Two-way sensitivity analysis was also performed to evaluate the sensitivity to the changes of variables such as medicine cost and effectiveness (Figure 3).The results showed that with increase of the effectiveness or decrease of FEIBA cost within the first hour of treatment, FEIBA can be the cost-effective strategy, but in the area with the increase of FEIBA cost, AryoSeven was more cost-effective.
Discussion
The current study was the first pharmacoeconomics study to compare the cost-effectiveness of bypassing agents in Hemophilia A patients with inhibitors through clinical trial and decision analytic model in Iran. It was previously reported that bypassing agents are effective to control bleeding episodes in patients with haemophilia and inhibitors (11-14). In recent years several biogeneric medicines received marketing authorization from Iran national regulatory authority (15). However, it is important to find out their cost effectiveness compared to the other brands in the market. The current study aimed to evaluate the cost-effectiveness of AryoSeven®-a Biogeneric rFVIIa in Iran market- compared with that of FEIBA® through clinical trial and decision analysis model. Cost of medicines, applied dosage, and also therapy success in the first line of the treatment were among the most important elements in the economic analysis, similar to other studies (16). In the study by Odeyemi et al. on bypassing agents modelling, in addition to medicine costs, hospitalization and transportation costs were also considered; considering the short time to control bleeding with rFVIIa compared to aPCC and less cost, it was generally less costly (17). The study had some limitations including inability to be performed blindly. However other studies such as the one by FENOC (2) were also performed as an open label study. It should be noted that the impact of patients` prior tendency to the type of products on the final effect of drugs, helped us to partially overcome the lack of blinding. Accordingly the results showed that prior tendency had no influence when the patients were treated with AryoSeven® but it did with FEIBA®, which may be due to their different attitudes towards original and generic products of rFVIIa. As mentioned in the previous sections and according to the review by Golestani et al. (18) although both drugs were effective none of them was permanently effective to treat joint bleeds. According to the results of the current study, the effectiveness of AryoSeven® and FEIBA® were 72% and 89%, respectively. However AryoSeven® was a more cost effective medicine compared to FEIBA® to manage bleeding in patients, although both strategies were undominated. The results of the current study were in line with those of the studies in which rFVIIa was introduced as the most cost-effective option comparing medicinal costs regarding bleeding episodes in patients with Haemophilia A with bypassing agents in the first line of the treatment (19,20). Although the results of the remodelling showed that if the price of FEIBA reduced by 25% it will be a more cost-effective strategy. To determine the average cost per bleeding, Stephens et al. (1) expressed that rFVIIa with US$ 28,076 had the lowest cost in the third line treatment compared to aPCC in the first line of treatment due to unnecessary use of rFVIIa in the second and third line treatments. However, there are other studies which showed cost-effectiveness of FEIBA® (3, 6). Different results may be due to different local cost of products, different dosage, and protocol of treatments and difference in assessment methods.
Conclusion
Although the obtained results showed the cost-effectiveness of AryoSeven®, both strategies were undominated. On the other hand FEIBA was more effective. Hence, both medicines can be applied in the first line of the treatment if the cost of FEIBA was reduced. Results of the current study can be considered for healthcare policy makers to select the best therapeutic strategies to be applied in the first line of treatment of patients with Haemophilia A and inhibitors. It is important to emphasize that based on the results of the current study, due to higher efficacy of FEIBA, policy makers could decide to reduce FEIBA price to the extent that both medicines would be equally considered cost-effective. In this case both therapies would be available to manage bleeding in patients.
Competing Interests
This study received a research grant from the Aryogen Biopharmaceutical Company, without any role or influence on design, administration, analysis, writing and submission of this article. Some of the authors received travel grants from Baxter, CSL, Octapharma, Novo Nordisk, and Aryogen companies.
Authors' Contributions
Study design, data collection and design of decision analysis model: MG; clinical trial design and evaluation of treatment effectiveness: PE; analysis of the cost-effectiveness and ICER ratio: AMC; all data supervision and study design review: HRR; review of clinical trial and data: JS; in clinical trial contribution: RH, BH; evaluation of the effectiveness of the treatment: MN, MRM, HH and GHT, review of the model and data analysis: AI; statistical analysis conduction: MTK. The final version of manuscript was approved by all authors.
Acknowledgements
References
-
1.
Stephens JM, Joshi AV, Munro V, Mathew P, Botteman MF. Pharmacoeconomic analysis of recombinant factor VIIa vs. APCC in the treatment of minor-to moderate bleeds in hemophilia patients with inhibitors. Curr. Med. Res. Opin. 2006;22:23-31.
-
2.
Astemark J, Donfield SM, DiMichele DM, Gringeri A, Gilbert SA, Waters J, Berntorp E, FENOC Study Group. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546-51. [PubMed ID: 16990605].
-
3.
Putnam KG, Bohn RL, Ewenstein BM, Winkelmayer WC, Avorn J. A cost minimization model for the treatment of minor bleeding episodes in patients with haemophilia A and high-titre inhibitors. Haemophilia. 2005;11:261-9. [PubMed ID: 15876272].
-
4.
Faranoush M, Abolghasemi H, Toogeh Gh, Karimi M, Eshghi P, Managhchi M, Hoorfar H, Keikhaei B, Dehdezi A, Mehrvar A, Khoeiny B, Kamyar K, Heshmat R, Baghaeipour MR, Mirbehbahani NB, Fayazfar R, Ahmadinejad M, Naderi M. A Comparison between recombinant activated factor VII (Aryoseven) and Novoseven in patients with congenital factor VII deficiency. CLIN APPL THROMB HEMOST. 2014.
-
5.
Di Minno MND, Di Minno G, Di Capua M, Cerbone AM, Coppola A. Cost of care of haemophilia with inhibitors. Haemophilia. 2010;16:190-201.
-
6.
Steen Carlsson K, Astermark J, Donfield S, Berntorp E. Cost and outcome: comparisons of two alternative bypassing agents for persons with haemophilia A complicated by an inhibitor. Thromb. Haemost. 2008;99:1060-7. [PubMed ID: 18521509].
-
7.
Khatibi M, Rasekh HR, Shahverdi Z, jamshidi HR. Cost-Effectiveness Evaluation of Quadrivalent Human Papilloma Virus Vaccine for HPV-Related Disease in Iran. Iran. J. Pharm. Res. 2014;13(supplement):225-234. [PubMed ID: 24711850].
-
8.
Escobar MA. Health economics in haemophilia: a review from the clinician’s perspective. Haemophilia. 2010;16:29-34. [PubMed ID: 20586799].
-
9.
Kavakli K, Makris M, Zulfikar B, Erhardtsen E, Abrams ZS, Kenet G. Home treatment of haemarthroses using a single dose regimen of recombinant activated factor VII in patients with haemophilia and inhibitors A multi-centre, randomised, double-blind, cross-over trial. Thromb. Haemost. 2006;95:600-5. [PubMed ID: 16601828].
-
10.
TreeAge Pro 2011 User's Manual. TreeAge Pro. 2011.
-
11.
Negrier C, Goudemand J, Sultan Y, Bertrand M, Rothschild C, Lauroua P. Multicenter retrospective study on the utilization of FEIBA in France in patients with factor VIII and factor IX inhibitors. Thromb. Haemost. 1997;77:1113-9. [PubMed ID: 9241742].
-
12.
Parameswaran R, Shapiro AD, Gill JC, Kessler CM. Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry. Haemophilia. 2005;11:100-6. [PubMed ID: 15810910].
-
13.
Key NS, Aledort LM, Beardsley D, Cooper HA, Davignon G, Ewenstein BM, Gilchrist GS, Gill JC, Glader B, Hoots WK, Kisker CT, Lusher JM, Rosenfield CG, Shapiro AD, Smith H, Taft E. Home treatment of mild to moderate bleeding episodes using recombinant factor VIIa (Novoseven) in haemophiliacs with inhibitors. Thromb. Haemost. 1998;80:912-8. [PubMed ID: 9869160].
-
14.
Hilgartner MW, Knatterud GL. The use of factor eight inhibitor bypassing activity (FEIBA Immuno) product for treatment of bleeding episodes in hemophiliacs with inhibitors. Blood. 1983;61:36-40. [PubMed ID: 6401216].
-
15.
Cheraghali AM. Biosimilars; a unique opportunity for Iran national health sector and national pharmaceutical industry. DARU. 2012;20:35. [PubMed ID: 23351613].
-
16.
Dimichele D, Negrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia. 2006;12:352-62. [PubMed ID: 16834734].
-
17.
Odeyemi I, Guest J. Modelling the economic impact of recombinant activated Factor VII compared to activated prothrombin-complex concentrate in the home treatment of a mild to moderate bleed in adults with inhibitors to clotting factors VIII and IX in the UK. J. Med. Econ. 2002;5:119-133.
-
18.
Golestani M, Eshghi P, Rasekh HR, Cheraghali AM, Salamzadeh J, Imani A. Comparison of bypassing agents in bleeding reduction in treatment of bleeding episodes in patients with haemophilia and inhibitors. Iran Red. Crescent Med. J. 2014;16:e24551. [PubMed ID: 25763245].
-
19.
Dundar S, Zülfikar B, Kavakli K, Gönen C, Zülfikar H, Yilmaz D, Hart WM, Sumen A, Tuna S, Karamalis M. A cost evaluation of treatment alternatives in mild-to-moderate bleeding episodes in haemophilia patients with inhibitors in Turkey. J. Med. Econ. 2005;8:45-54.
-
20.
Ozelo MC, Villaca PR, De Almeida JOSC, Bueno TMF, DE Miranda PAP, Hart WM, Karamalis M. A cost evaluation of treatment alternatives for mild-to-moderate bleeding episodes in patients with haemophilia and inhibitors in Brazil. Haemophilia. 2007;13:462-9. [PubMed ID: 17880430].