Abstract
Keywords
Platelet PRP VASP Pre-analytical variation Flow cytometry Quality control
Introduction
Vasodilator-stimulated phosphoprotein (VASP) is a regulator of actin reorganization in platelets. VASP-actin interaction prevents capping proteins from binding to actin, therefore allows growing of the actin polymers (1). Phosphorylation of VASP at different amino acid residues such as Serine 157 (Ser157) and Serine 239 (Ser239) decreases the affinity of this molecule for binding to actin, leading to inhibition of actin reorganization in platelets (2). Platelet inhibitors such as forskolin, prostaglandin E1 (PGE1), sodium nitro-prusside (SNP) exert their effects through the stimulation of VASP phosphorylation (3).
Considering VASP as a common downstream target of various signaling pathways, an increasing attention to this molecule has been taken in platelet studies (4, 5). Likewise, in clinical laboratories, flow cytometry or enzyme linked immunosorbent assay (ELISA)-based phospho-VASP (P-VASP) analysis has been increasingly applied as an in-vitro approach for monitoring of anti-platelet therapy with adenosine diphosphate (ADP) receptor antagonists (6, 7). But having said that, whether applying P-VASP analysis in clinical laboratories for this purpose provides a proper degree of correlation and/or agreement with other approaches, has been a matter of serious discussions (-).
The validity of platelet experiments may be adversely influenced by inter- and intra-test variations. The procedures in pre-analytical phase of experiments are probably the most important sources of variations in platelet assessments (12, 13). The effect of washing step to induce platelet activation has been well described before (14). Even a small deviation of platelet from the physiological state of activity has a great influence on its responses to the experimental treatments (15). Considering the role of VASP phosphorylation in controlling of platelet activation, the question which may be raised is how much P-VASP dynamics in platelets can be affected by variations in pre-analytical sample preparations? No controlled study has been found that evaluated possible effects of those variations on intraplatelet P-VASP expression. The aim of this study was comparing the intraplatelet P-VASP expression between differently handled platelet samples. Therefore, to this purpose, platelet rich plasma (PRP) and washed platelet samples were subjected to comparative evaluations.
Experimental
Preparation of PRP and washed platelets
According to the ethical committee guidelines of our institute, blood samples were obtained with consent from healthy donors who denied taking anti-platelet drugs for at least two weeks in plastic tubes containing sodium citrate 3.8 %. PRP was prepared from whole blood samples according to our in house protocol (16). Each PRP sample was divided into halves and washing procedure was undertaken for one part, by adapting the procedure used by others (17, 18). In order to washing, the PRP sample was diluted in citrate wash buffer (11 mM glucose, 128 mMNaCl, 4.3 mM NaH2PO4, 7.5 mM Na2HPO4, 4.8 mM sodium citrate, 2.4 mM citric acid, pH 6.5) containing freshly added 50 u/mL heparin. After centrifugation at 800 x g for 10 min at 25 °C, supernatant was removed and the pellet was rinsed without resuspension by gently adding of citrate wash buffer and removing slowly. This type of rinsing step was applied to improve recovery of platelet number at the end of the procedure. Then modified Tyrode’s-HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer containing 0.02 u/mL apyrase was added on platelet pellet. The sample was then incubated for 15 min at 37 °C before resuspension. The concentration of platelets in washed samples was adjusted to the equal level of PRP samples and allowed to rest at least for 1 hour at 25 °C prior to be used in experiments.
Experimental treatments on washed and PRP samples
PRP and washed platelet samples were incubated in the presence of 50 nM PGE1 (Sigma), 10 µM forskolin (Calbiochem), or 100 µM SNP (Merck). Untreated PRP and washed platelet samples were also included in each run of the experiments as baseline controls. After 10 min incubation at room temperature on shaker, the treatments were terminated by adding Para-formaldehyde (1% final concentration).
Flow cytometry analysis
The platelet samples which were fixed in Para-formaldehyde, permeabilized in 0.5% Triton X-100 in PBS for 30 min at 4 °C. Samples containing 20x106 platelets were then incubated with a specific primary antibody (1/200 in 2% bovine serum albumin, BSA, in PBS) for 14 hour at 4 °C with gentle shaking. Primary antibodies utilized were polyclonal rabbit anti-P-Ser157-VASP and polyclonal rabbit anti-P-Ser239-VASP (from Cell Signaling). After a washing step, FITC-conjugated goat anti-rabbit polyclonal IgG (1/500, Santa Cruz Biotechnology) was used as secondary antibody. Flow cytometric analysis of 10,000 events in platelet area / sample was performed using a flow cytometer (CyFlow®Space, Partec GmbH, Germany). Platelets were gated by forward (FSC)/ side (SSC) scatter characteristics, the median fluorescence intensity (MFI) was quantified for gated events using Flomax instrument software. In each run of experiments, a platelet sample labeled only with secondary antibody was included as negative control to exclude the fluorescence due to non-specific binding of secondary antibodies.
After each run of experiments on either PRP or washed platelet samples, MFI value obtained for untreated sample was considered as baseline and the percent of MFI shift from baseline, representing the percent of induced change in P-VASP expression, for a forskolin-, PGE1-, or SNP-treated sample was calculated, using formula
Statistical analysis
Statistical significance was analyzed using non-parametric analysis of variance.
Results and Discussion
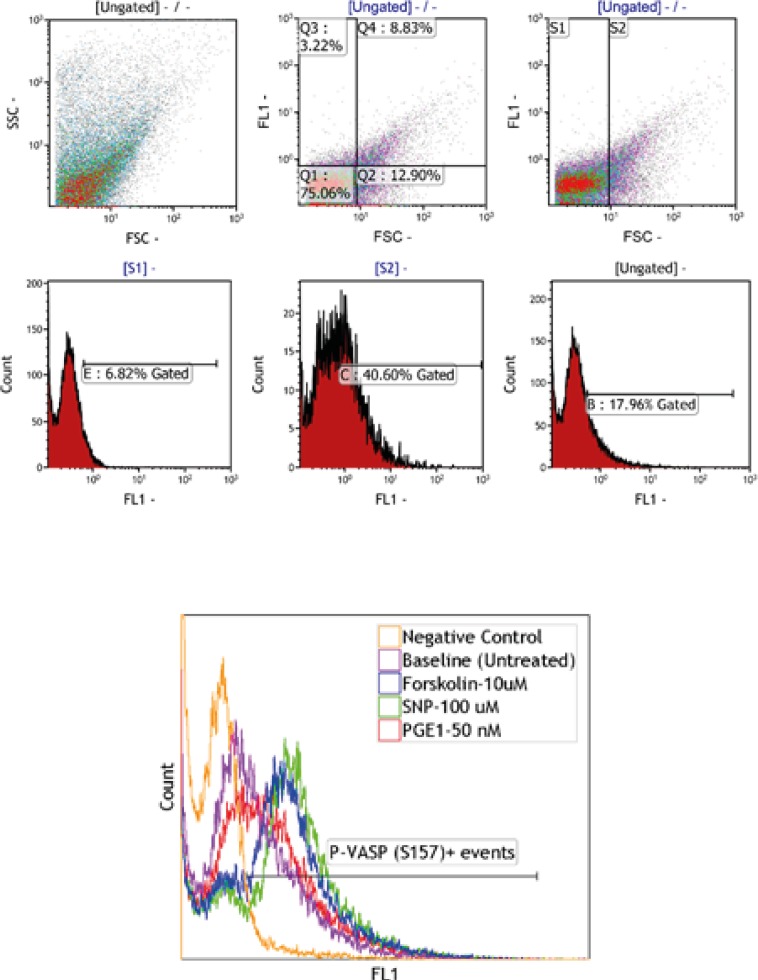
PRP and washed platelet samples, labeled by anti-P-ser157-VASP and anti-P-ser239-VASP, were subjected to flow cytometric analysis. The gating strategy which was applied has been described in Figure 1. Fluorescence intensity histograms from one representative experiment out of six performed is shown in Figure 2. The histograms obtained from conducting the P-VASP analysis on PRP and washed platelet, indicates different behavior of PRP and washed platelets to express intracellular P-VASP at baseline state and/or in response to different inducers of VASP phosphorylation.
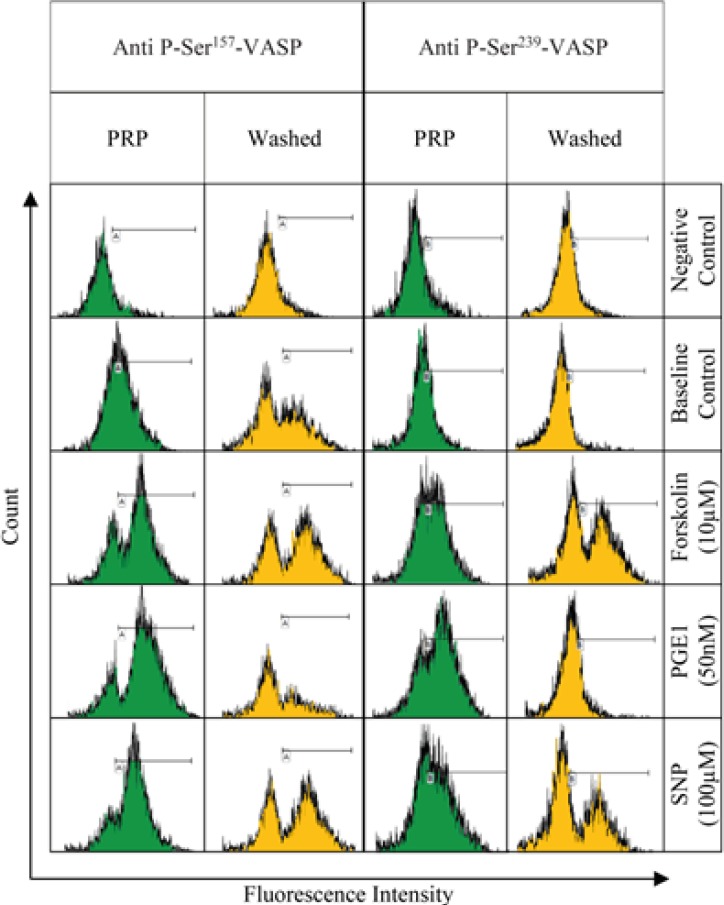
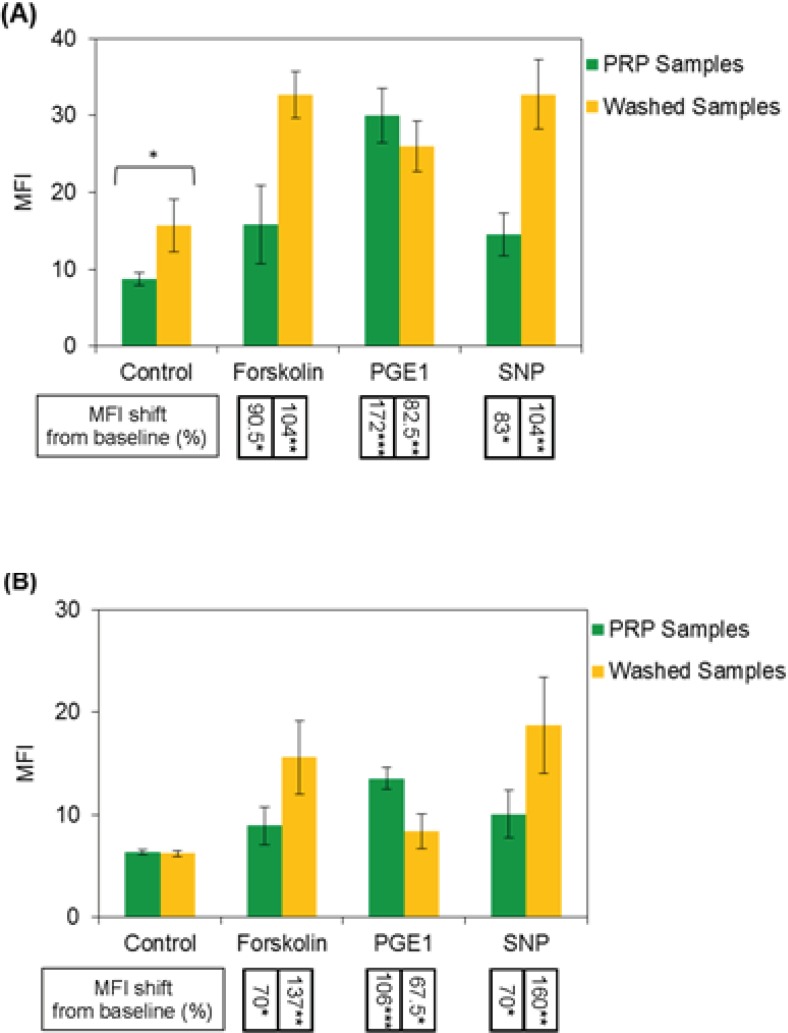
Expressions of intraplatelet P-ser157-VASP and P-ser239-VASP were quantified by flow cytometry software, as median fluorescence intensity value (MFI). From the graphs in Figure 3, the MFI values obtained for PRP and washed platelet samples could be compared. The results, as shown in graph (A), indicate more expression of P-Ser157-VASP at baseline level for washed platelets, however, no significant differences were found between two types of the samples in expression of P-Ser239-VASP at their baseline levels, graph (B). The graphs also indicate more levels of forskolin- and SNP-induced VASP phosphorylation at Serine 157, 239 for washed platelets, whereas, reduced levels of PGE1-induced VASP phosphorylation at both residues were detected for washed platelets.
Research and clinical laboratory experiments on platelets may be conducted on whole blood, PRP, or washed platelet samples(19). Variation in pre-analytical processing of samples may cause considerable morphological and functional changes in platelets. To avoid such variations and also save time and labor, clinical laboratories prefer to conduct platelet experiments on less handled samples. Likewise, analyzing of intraplatelet VASP phosphorylation on whole blood samples using ELISA- or flow cytometry-based standardized kits has been considered by clinical laboratories. In spite of this, several possible variations such as those associated with blood sampling remain to be controlled (20). Therefore despite using the kits, performing of intraplatelet P-VASP analysis in a clinical laboratory still needs a high level of experience and proficiency. On the other hand, washed platelets may be preferably chosen to be applied in research studies, to ensure that the effects of matrix variables on obtained results are excluded. We hope our findings provide a better understanding about the changes, that might be occur in intraplatelet P-VASP expression due to variation in pre-analytical sample preparation. Considering a proper approach for dealing with those changes may help improving of validity and reproducibility of P-VASP measurements, both in clinical and research investigations.
The present study was designed to evaluate intraplatelet VASP phosphorylation by flow cytometry method. Specific interactions of applied antibodies in flow cytometric analysis of phosphorylated VASP had been confirmed by western blot analysis, before using in experiments (data not shown). Our results indicated more levels of P-Ser157-VASP expression at baseline for washed platelets, compared with the platelets existed in PRP samples. The elevation of Ser157-VASP expression in thrombin and collagen activated platelets has been also reported, previously (21, 22). Platelets are highly susceptible to be priming along with in-vitro manipulations. Such an artifactual pre-activation of platelets can be started from the time of blood sampling and progress with further manipulations, but under controlled condition this status is usually reversible and platelets tend to return to their resting phenotype again (23). In this study high attention was taken to prevent the progress of platelet activation during washing procedure and while platelet endured some reversible shape changes after washing steps, the monitoring of platelets after washing procedure revealed no significant increases in P-selectin expression.
The results from this study showed no significant differences in the levels of P-Ser239-VASP expression at baseline state between washed and PRP samples. This finding is consistent with the data from previous studies, indicating unchanged levels of P-Ser239-VASP in agonist activated platelets (21).
Although Ser157 residue on VASP molecule is the primary target of phosphorylation by PGE1, commonly used flow cytometry kits available for monitoring of anti-P2Y12 drugs, instead evaluate the levels of P-Ser239-VASP expression. It seems reasonable approach; because according to our findings, variation in the pre-analytical sample preparations may cause fewer changes in baseline expression of P-Ser239-VASP in platelets, compared with those of Ser157 residue, graph B in Figure 2.
After treatment of washed and PRP samples in the presence of different P-VASP inducers, washed platelets revealed more levels of forskolin- and SNP-induced VASP phosphorylation but less extents of PGE1-induced P-VASP expression, compared with the platelets in PRP samples. Forskolin and PGE1 are known to stimulate intraplatelet VASP phosphorylation by similar mechanism, which is inducing of cAMP (cyclic Adenosine Monophosphate) cascade (24). In spite of this, washing procedure was able to modulate their effects in opposite directions; this might be explained by releasing of some contents of endogenous ADP from manipulated platelets in experimental environment. It may worth mentioning that susceptibility of PGE1-mediated adenylate cyclase activation to be reversed in the presence of ADP has been established before (25, 26).
Variability of intraplatelet P-VASP expression observed between PRP and washed platelet samples might be related to the mechanical stress, which could be exerted on platelets by centrifugation and other procedures of washing step, however, possible effects of matrix components within different group of samples cannot be also ignored. One other caveat that must be noted here is that the results from this study may have been influenced by test-specific properties of applied method (flow cytometry). Further investigations using ELISA-based P-VASP analysis are required to be undertaken for estimating the effect of those factors.
Conclusion
Our results suggested that the dynamics of phospho-VASP expression in platelets is sensitive to be changed due to variation in pre-analysis samples preparations. The Patterns of observed changes were different, regarding the type of P-VASP inducer and also the amino acid residue of phosphorylation on VASP molecule. The data provided from this study might be considered as an integral part of background information that is required for performing of intraplatelet P-VASP analysis and interpretation of the results. An extensive attention to avoid platelet stimulation during blood sampling and processing might be important to prevent artifactual variations of P-VASP data in platelet pharmacology.
Acknowledgements
References
-
1.
Bearer EL, Prakash JM, Manchester RD, Allen PG. VASP protects actin filaments from gelsolin: an in-vitro study with implications for platelet actin reorganizations. Cell Motil. Cytoskeleton. 2000;47:351-364. [PubMed ID: 11093254].
-
2.
Bearer EL, Prakash JM, Li Z. Actin dynamics in platelets. Int. Rev. Cytol. 2002;217:137-182. [PubMed ID: 12019562].
-
3.
Thijs T, Nuyttens BP, Deckmyn H, Broos K. Platelet physiology and antiplatelet agents. Clin. Chem. Laborator. Med. 2010;48:3-13.
-
4.
Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, Renné T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J. Cell Sci. 2009;122:3954-3965. [PubMed ID: 19825941].
-
5.
Aszodi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37-48. [PubMed ID: 9878048].
-
6.
Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J. Thromb. Haemost. 2005;3:85-92. [PubMed ID: 15634270].
-
7.
Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thrombosis and Haemostasis. 1999;82:1145-1152. [PubMed ID: 10494779].
-
8.
Hezard N, Metz D, Garnotel R, Simon G, Mace C, Koebel P, Nguyen P. Platelet VASP phosphorylation assessment in clopidogrel-treated patients: lack of agreement between Western blot and flow cytometry. Platelets. 2005;16:474-481. [PubMed ID: 16323337].
-
9.
Cuisset T, Frere C, Poyet R, Quilici J, Gaborit B, Bali L, Brissy O, Lambert M, Morange PE, Alessi MC, Bonnet JL. Clopidogrel response: head-to-head comparison of different platelet assays to identify clopidogrel non responder patients after coronary stenting. Arch. Cardiovas. Dis. 2010;103:39-45.
-
10.
Bal Dit Sollier C, Berge N, Boval B, Dubar M, Drouet L. Differential sensitivity and kinetics of response of different ex-vivo tests monitoring functional variability of platelet response to clopidogrel. Thrombosis and Haemostasis. 2010;104:571-581. [PubMed ID: 20664906].
-
11.
Haji Aghajani M, Kobarfard F, Safi O, Sheibani K, Sistanizad M. Resistance to Clopidogrel among Iranian Patients Undergoing Angioplasty Intervention. Iran. J. Pharm. Res. 2013;12:169-174. [PubMed ID: 24250685].
-
12.
Stegnar M, Knezevic A, Bozic-Mijovski M. The effect of pre-analytical variables on light transmittance aggregometry in citrated platelet-rich plasma from healthy subjects. Clin. Chem. Laboratory Med. 2010;48:1463-1465.
-
13.
Hayward CP, Eikelboom J. Platelet function testing: quality assurance. Thrombosis and Hemostasis. 2007;33:273-282.
-
14.
Veeraputhiran M, Ware J, Dent J, Bornhorst J, Post G, Cottler-Fox M, Pesek G, Theus J, Nakagawa M. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51:1030-1036. [PubMed ID: 20946201].
-
15.
Wada K, Takiguchi Y, Nakashima M. Changes in platelet aggregation in whole blood, plasma and washed platelets in streptozotocin-induced diabetic rats: time-dependent change in the antiaggregatory activity of diabetic rat plasma. Platelets. 1993;4:280-284. [PubMed ID: 21043752].
-
16.
Aliasgharzadeh A, Gharehbaghian A, Taherian AA, Ghasemzadeh M, Salimian M. Modulation of hyperthermia-induced platelet aggregation inhibition in the presence of urea. Int. J. Hyperthermia. 2013;29:256-258. [PubMed ID: 23517402].
-
17.
Ghasemzadeh M, Kaplan ZS, Alwis I, Schoenwaelder SM, Ashworth KJ, Westein E, Hosseini E, Salem HH, Slattery R, McColl SR, Hickey MJ, Ruggeri ZM, Yuan Y, Jackson SP. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555-4566. [PubMed ID: 23550035].
-
18.
Cazenave JP, Ohlmann P, Cassel D, Eckly A, Hechler B, Gachet C. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol. Biol. 2004;272:13-28. [PubMed ID: 15226531].
-
19.
Faghih Akhlaghi M, Amidi S, Esfahanizadeh M, Daeihamed M, Kobarfard F. Synthesis of N-arylmethyl substituted indole derivatives as new antiplatelet aggregation agents. Iran. J. Pharm. Res. 2014;13:35-42. [PubMed ID: 24711827].
-
20.
B EK, M FB. State of the art in platelet function testing Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur. Transfus. Med. Hemother. 2013;40:73-86. [PubMed ID: 23653569].
-
21.
Gambaryan S, Kobsar A, Rukoyatkina N, Herterich S, Geiger J, Smolenski A, Lohmann SM, Walter U. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NFkappaB-IkappaB complex. J. Biol. Chem. 2010;285:18352-18363. [PubMed ID: 20356841].
-
22.
Wangorsch G, Butt E, Mark R, Hubertus K, Geiger J, Dandekar T, Dittrich M. Time-resolved in silico modeling of fine-tuned cAMP signaling in platelets: feedback loops, titrated phosphorylations and pharmacological modulation. BMC Syst. Biol. 2011;5:178. [PubMed ID: 22034949].
-
23.
Harrison P, Mackie I, Mumford A, Briggs C, Liesner R, Winter M, Machin S. Guidelines for the laboratory investigation of heritable disorders of platelet function. British J. Haematol. 2011;155:30-44.
-
24.
Feinstein MB, Egan JJ, Sha'afi RI, White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem. Biophys. Res. Communicat. 1983;113:598-604.
-
25.
Mellwig KP, Jakobs KH. Inhibition of platelet adenylate cyclase by ADP. Thrombosis Res. 1980;18:7-17.
-
26.
Alvarez R, Taylor A, Fazzari JJ, Jacobs JR. Regulation of cyclic AMP metabolism in human platelets Sequential activation of adenylate cyclase and cyclic AMP phosphodiesterase by prostaglandins. Molecul. Pharmacol. 1981;20:302-309.