Abstract
Keywords
Introduction
Runx (Runt-related) family of genes is DNA-binding and cancer related transcription factors that regulate the cell growth, proliferation and differentiation in humans (1, 2). The three members of this family are important targets of transforming growth factor β (TGF-β) super family signaling and play critical functions in mammalian development (3). Runx proteins have shown to interact with downstream SMAD (small mothers against decapentaplegic) proteins in mediating the growth-suppressive effects of TGF-β (4). Although all three members of Runx family share highly conserved DNA-binding domains (128 amino acid regions designated as runt domain), they regulate distinct functions and play important roles in both normal developmental processes and carcinogenesis (5-7). Runx1 is involved in hematopoiesis, angiogenesis and human acute leukemia (8) and Runx2 is essential for bone and tooth development and involved in cleidocranial dysplasia (9).
Runx3, the smallest member of Runx family, is a putative tumor suppressor gene located at chromosome 1p36 and is critical for gastric epithelial differentiation, neurogenesis of dorsal root ganglia and T cell differentiation (10, 11). In addition, Runx3 is deeply involved in various cancer processes such as cell growth, apoptosis, angiogenesis, and metastasis (12). Previous studies strongly suggested that Runx3 is a tumor suppressor in various carcinomas, including gastric and breast carcinoma and its loss is related to frequently inactivation by dual mechanism of protein cytoplasmic mislocalization and promoter hypermethylation (10, 12-15). Interestingly, the oncogenic potential of Runx3 has also been observed in some cases, such as head and neck and ovarian cancer, due to the demethylation and/or cytoplasmic mislocalization (12, 16, 17).
Runx3 plays an important role in inhibiting cellular growth by participating in the TGF-β signaling pathway. It has been shown that Runx3 is responsible for transcriptional up-regulation of Bim in TGF-β-induced apoptosis (14, 18). Therefore, expression of exogenous Runx3 and study of its effect on cell proliferation and cell death can provide important information to develop anticancer agents and gene therapy strategies as well as better diagnosis.
In this study, we have evaluated the over-expression effect of Runx3 on proliferation and viability of cells with undetectable Runx3 expression (AGS) and low Runx3 expression (A549). Furthermore, we tested the effect of Runx3 expression on mRNA expression of Bax in these cells.
Experimental
Cell culture and transfection
The human lung cancer cell line A549 and human gastric adenocarcinoma cell line AGS (Pasteur Institute Cell Bank, Iran) were grown in RPMI-1640 medium (Euroclone, EU) supplemented with 10% fetal bovine serum (Gibco, UK) and 1% antibiotic penicillin-streptomycin (Biosera, UK) in 5% CO2 at 37°C incubator.
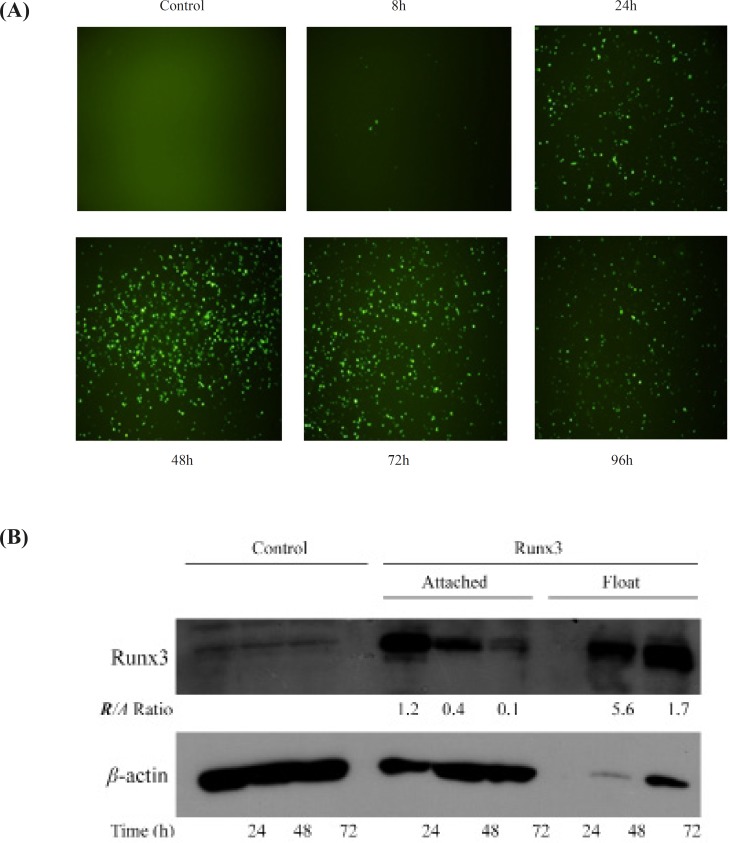
Human Runx3 complementary DNA (cDNA) (NM_004350) was subcloned into pReceiver-EGFP vector (Genecopoeia, USA) and transiently transfected into A549 and AGS cell lines. Cells were plated 24 h before transfection to reach 70-90% confluency. After 24 h, A549 cells and AGS cells were transfected with Fugene® 6 reagent (Roche, Germany) and PolyFect® transfection reagent (Qiagen, Germany), respectively, according to the instruction manual. The untransfected cells and the cells transfected with empty vector were used as negative controls. The transfected cells were analyzed for Runx3 protein expression using EGFP (enhanced green fluorescent protein) tag by fluorescent microscopy at various time points. The Runx3 gene expression was further analyzed using western blot analysis.
Western blot analysis
To detect Runx3 protein expression, the cells were transfected and cell lysates were prepared from attached and float cells 24, 48 and 72 h after the transfection. Standard western blotting was done with a polyclonal antibody against human Runx3 (Santa Cruz, USA).
Cell proliferation assays
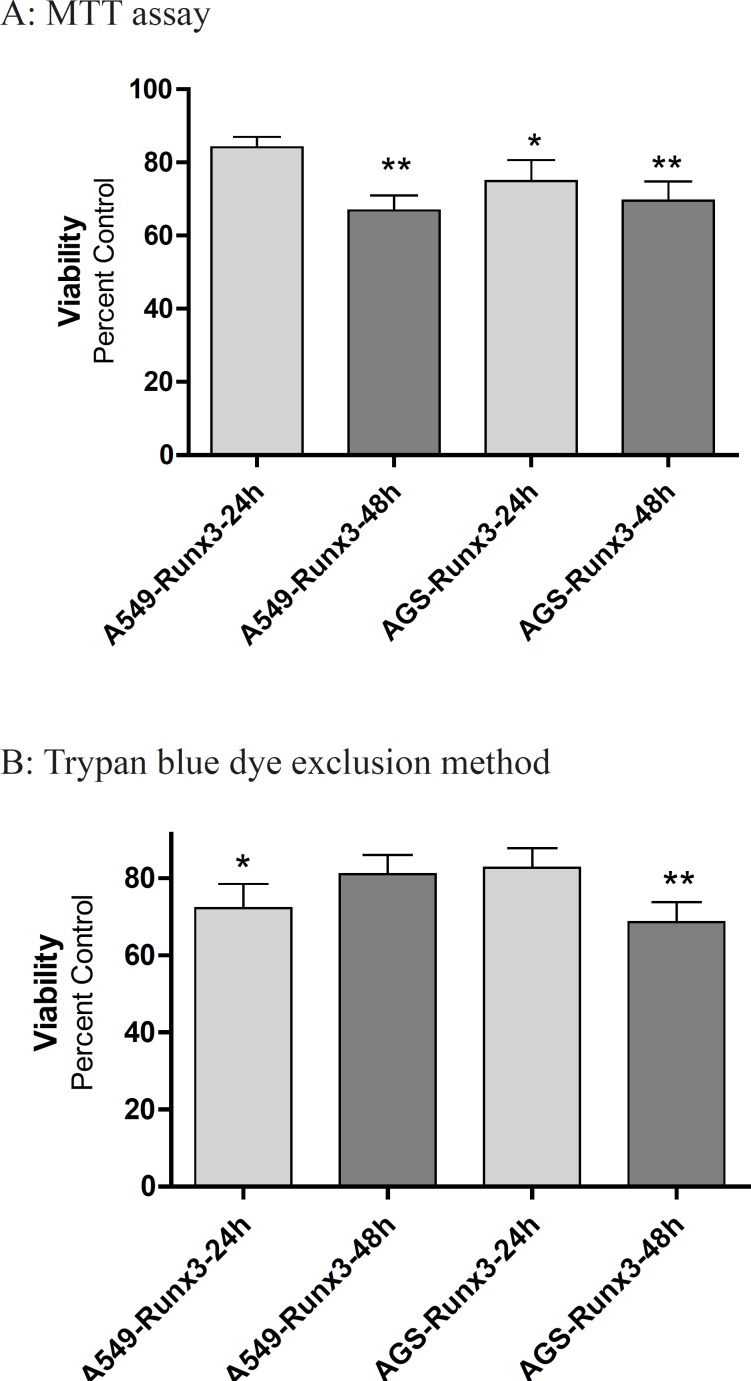
The cell proliferation and cell viability were evaluated 24 and 48 h after transiently transfecting using Methyl tetrazolium cytotoxicity assay (MTT, Sigma, UK) and Trypan blue dye exclusion method. For measuring cell viability, cells were plated in 96-well plates at density of 2.5x104 cells/well in triplicate. Cells were incubated, 24 and 48 h after the transfection, with MTT solution (3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, 0.5 mg/mL) for 4 h at 37°C. After the incubation period, the formazan crystals were dissolved in DMSO. The optical density (OD) was measured on microplate reader (BioTek, USA) at 570 nm, with 690 nm as a reference wavelength. The change in viability was calculated as percent viability compared to control group and the results of the three independent experiments were presented as mean ± SE (n = 3).
To evaluate the number of viable cells, the cells were seeded at a density of 1×105 cells/well into 24-well culture plates in triplicate. The number of live cells in each well was counted at 24 and 48 h after transfection using 0.4% trypan blue dye (Sigma, USA). The change in live cells was calculated as control percentage and results of the three independent experiments were presented as mean ± SE (n = 3).
Semiquantitative analysis of Bax MRNA levels by RT-PCR
Total RNA was extracted from A549 and AGS cells 24 h after transfection using TriPure Isolation Reagent® (Roche, Germany) and cDNA was synthesized from 2 µg of total RNA by using RevertAid® H Minus Kit (Fermentas, EU ) according to the manufacturer’s manual.
BCL2-associated X protein (Bax) mRNA (Messenger RNA) expression was measured by the semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) at different concentrations of cDNA (0.02, 0.08 and 0.16 µg/µL) using 5′- CAGCTCTGAGCAGATCATGAAGAC -3′ (sense) and 5′- GCCCATCTTCTTCCAGATGGTGAGC -3′ (antisense) primers, which yielded a 535 bp PCR product (19). The PCR was performed using the following program: 95°C for 2 min, 35 cycles of denaturation at 95°C for 30 sec, annealing at 64°C for 30 sec, and extension at 7°C for 40 sec and a final extension at 72°C for 5 min. The β-actin was used as house-keeping gene internal control (20). The PCR products were analyzed on 1% agarose gels and UV illumination. The bands were digitized using Scion-Image software (Scion Corporation, USA). The expression ratio was calculated based on the band intensity of targeted gene to β-actin.
Statistical analysis
Data presented as the means ± SE to at least three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Tukey-Kramer’s multiple comparison and p-values less than 0.05 were considered as significant.
Results
Effect of Runx3 expressionin AGS and A549 cell proliferation
Transient transfection of Runx3 indicated the protein expression within 24 h intransfected cells confirmed by microscopic analysis of EGFP tag (Figure 1A). Furthermore, Western blotting analysis indicated the high protein expression of Runx3 after transfection (Figure 1B). Interestingly, most of the AGS cells expressing GFP -Runx3 were dead and floating. The western blot analysis indicated the over-expression of GFP- Runx3 in AGS cells. The expression was higher in dead floating cells compared to the attached cells (Figure 1B) . In A549 cells, unlike AGS cells, fewer transfected cells expressing GFP were detected. This difference can be due to the cell line variation, protein expression and/or the timing of cell death in these cells. As indicated , the expression of Runx3 washighin 24-72 hand decreased in 96 h due to the death of expressing cells (Figure 1A).
The transfection of GFP-Runx3 inhibited proliferation of AGS and A549 cells within 48 h (Figure 2). According to the MTT results, AGS cells over expressing Runx3 showed 30-40% lower viability within 48 h compared to the vector transfected (Control) cells (p < 0.01; Figure 2A). These results were confirmed by Trypan blue dye exclusion method where 31.4% decrease in live cells were observed in 48 h after the transfection (p < 0.01). In A549 cells, a significant decrease in viability (33.2%, p < 0.01) was observed in 48 h based on the MTT results (Figure 2A).
RT-PC Ranalysis of Baxm RNA expression
To investigate the effect of Runx 3 transfection on Baxm RNA expression, we evaluated the expression of these genes by RT-PC Ranalysis. As shownin Figure 3, Runx3 induced Bax expression in AGS cells (p< 0.05 ) ,suggesting induction of cell death in these cells.
Discussion
Runx family of proteins have essential functions in both cell proliferation and differentiation (1, 6) and play roles as proto-oncogenes and tumor suppressors (1). Among them, Runx3 is a tumor suppressor that has antiproliferative effect and induces TGF-β-dependent apoptosis (21).
In this study, we have tested the effect of Runx3 expression on proliferation of AGS cells with undetectable protein level of Runx3 and A549 cells with low expression of the protein. We presently show that Runx3 inhibits cell proliferation and viability in both AGS and A549 cells. These findings are in agreement with previous reports indicating that expression of Runx3 will restore TGF-β induced apoptosisin biliary track cancer cell, esophageal adenocarcinoma and AGS cells(21-23). It should be mentioned that in both A549 and AGS cells, Runx3, overexpressing by itself, induces cell death. Moreover, our results indicate that Runx3 induces Bax expression in AGS cells (Figure 3).
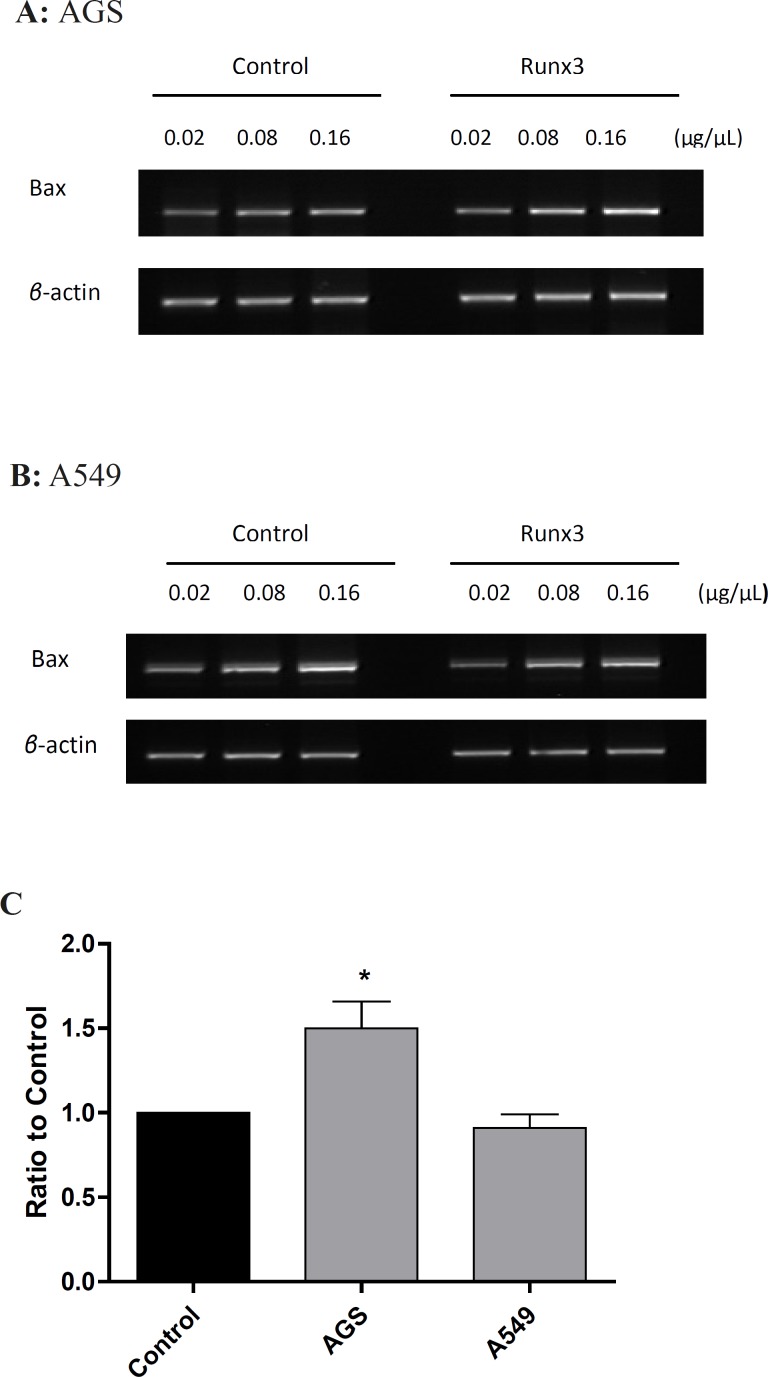
It is known that Bax can promote mitochondria-mediated apoptosis in AGS cells (24). Therefore, in AGS cells, Runx3-induced cell death can be mediated via Bax expression. On the other hand, Runx3 expression did not change Bax mRNA level in A549 cells (Figure 3). So far, there have been no reports on the effect of Runx3 expression on Bax induction. It has been reported that the over expression of Runx3 in gastric cancer cells down regulates Bcl-2 and directly activates the promoter activity of Bim to enhance TGF-β-dependent apoptosis, therefore, sensitizes cancer cells to chemotherapeutic drugs (18, 25). In addition, it has been reported that Runx2 induces Bax expression in osteosarcoma cells by binding to regulatory domain on the human Bax promoter (26). Hence, it is possible that Runx3 utilizes the same mechanism as Runx2 to induce Bax expression. However, this mechanism is cell dependent since in A549 cells, Runx3 expression could not alter Bax level and perhaps a more complex mechanism is involved in Runx3-induced Bax expression.
Taken together, Runx3 transfection inhibits cell proliferation in AGS and A549 cells. Moreover, Runx3 expression increases Bax mRNA levels in AGS cells indicating the induction of cell death and cell cycle arrest. However, Runx3 expression fails to induce Bax in A549 cells.
References
-
1.
Coffman JA. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol. Int. 2003;27:315-324. [PubMed ID: 12788047].
-
2.
Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-β superfamily and Runx proteins. Oncogene. 2004;23:4232-4237. [PubMed ID: 15156178].
-
3.
Ito Y. Molecular basis of tissue-specific gene expression mediated by the Runt domain transcription factor PEBP2/CBF. Genes to Cells. 1999;4:685-696. [PubMed ID: 10620014].
-
4.
Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-β superfamily signaling. Curr. Opin. Gen. Develop. 2003;13:43-47.
-
5.
Kagoshima H, Shigesada K, Satake M, Ito Y, Miyoshi H, Ohki M, Pepling M, Gergen P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genetics. 1993;9:338-341.
-
6.
Ito Y. Oncogenic potential of the RUNX gene family: ‘Over viow’. Oncogene. 2004;23:4198-4208. [PubMed ID: 15156173].
-
7.
Lund AH, Van Lohuizen M. RUNX: A trilogy of cancer genes. Cancer Cell. 2002;1:213-215. [PubMed ID: 12086855].
-
8.
Silva FPG, Morolli B, Storlazzi CT, Anelli L, Wessels H, Bezrookove V, Kluin-Nelemans HC, Giphart-Gassler M. Identification of RUNX1/AML1 as a classical tumor suppressor gene. Oncogene. 2003;22:538-547. [PubMed ID: 12555067].
-
9.
Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nature Genetics. 1997;16:307-310. [PubMed ID: 9207800].
-
10.
Li QL, Ito K, Sakakura C, Fukamachi H, Inoue KI, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [PubMed ID: 11955451].
-
11.
Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, Kremer E, Otto F, Brenner O, Lev-Tov A, Groner Y. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454-3463. [PubMed ID: 12093746].
-
12.
Subramaniam MM, Chan JY, Yeoh KG, Quek T, Ito K, Salto-Tellez M. Molecular pathology of RUNX3 in human carcinogenesis. Biochim. Biophys. Acta - Rev. Cancer. 2009;1796:315-331.
-
13.
Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, Hiong KC, Peh BK, Han HC, Ito T, Teh M, Yeoh KG, Ito Y. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005;65:7743-50. [PubMed ID: 16140942].
-
14.
Yamamura Y, Wei LL, Inoue KI, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J. Biol. Chem. 2006;281:5267-5276. [PubMed ID: 16373335].
-
15.
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito K, Inoue M, Putti TC, Loh M, Ko TK, Huang C, Bhalla KN, Zhu T, Ito Y, Sukumar S. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res. 2006;66:6512-6520. [PubMed ID: 16818622].
-
16.
Tsunematsu T, Kudo Y, Iizuka S, Ogawa I, Fujita T, Kurihara H, Abiko Y, Takata T. RUNX3 has an oncogenic role in head and neck cancer. PLoS ONE. 2009;4:e5892. [PubMed ID: 19521519].
-
17.
Nevadunsky NS, Barbieri JS, Kwong J, Merritt MA, Welch WR, Berkowitz RS, Mok SC. RUNX3 protein is overexpressed in human epithelial ovarian cancer. Gynecol. Oncol. 2009;112:325-330. [PubMed ID: 18937968].
-
18.
Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue KI, Ida H, Bouillet P, Strasser A, Bae SC, Ito Y. The RUNX3 tumor suppressor upregulates bim in gastric epithelial cells undergoing transforming growth factor β-induced apoptosis. Mol. Cell. Biol. 2006;26:4474-4488. [PubMed ID: 16738314].
-
19.
Kosmider B, Wojcik I, Osiecka R, Bartkowiak J, Zyner E, Ochocki J, Liberski P. Enhanced P53 and BAX gene expression and apoptosis in A549 cells by cis-Pt(II) complex of 3-aminoflavone in comparison with cis-DDP. Invest. New Drugs. 2005;23:287-297. [PubMed ID: 16012788].
-
20.
Schwaller J, Pabst T, Bickel M, Borisch B, Fey M, Tobler A. Comparative detection and quantitation of human CDK inhibitor mRNA expression of p15INK4B, p16INK4A, p16β, p18INK4C, p19INK4D, p21WAF1, p27KIP1 and p57KIP2 by RT-PCR using a polycompetitive internal standard. Brit. J. Haematol. 1997;99:896-900. [PubMed ID: 9432039].
-
21.
Torquati A, O’Rear L, Longobardi L, Spagnoli A, Richards WO, Daniel Beauchamp R. RUNX3 inhibits cell proliferation and induces apoptosis by reinstating transforming growth factor beta responsiveness in esophageal adenocarcinoma cells. Surgery. 2004;136:310-316. [PubMed ID: 15300196].
-
22.
Hasegawa K, Yazumi S, Wada M, Sakurai T, Kida M, Yamauchi J, Hisatsune H, Tada S, Ida H, Nakase Y, Sakakura C, Hagiwara A, Chiba T. Restoration of RUNX3 enhances transforming growth factor-beta-dependent p21 expression in a biliary tract cancer cell line. Cancer Sci. 2007;98:838-43. [PubMed ID: 17470130].
-
23.
Hsu PI, Hsieh HL, Lee J, Lin LF, Chen HC, Lu PJ, Hsiao M. Loss of RUNX3 expression correlates with differentiation, nodal metastasis, and poor prognosis of gastric cancer. Ann. Surg. Oncol. 2009;16:1686-1694. [PubMed ID: 19290488].
-
24.
Kim KM, Lee SG, Park MG, Song JY, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. γ-Glutamyltranspeptidase of Helicobacter pylori induces mitochondria-mediated apoptosis in AGS cells. Biochem. Biophys. Res. Commun. 2007;355:562-567. [PubMed ID: 17307146].
-
25.
Guo C, Ding J, Yao L, Sun L, Lin T, Song Y, Fan D. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int. J. Cancer. 2005;116:155-160. [PubMed ID: 15756676].
-
26.
Eliseev RA, Dong YF, Sampson E, Zuscik MJ, Schwarz EM, O’Keefe RJ, Rosier RN, Drissi MH. Runx2-mediated activation of the Bax gene increases osteosarcoma cell sensitivity to apoptosis. Oncogene. 2008;27:3605-14. [PubMed ID: 18223689].