Abstract
Keywords
Human serum albumin Fluoxetine Cortisol Spectroscopic methods pH meter
Introduction
Human serum albumin (HSA) is one of the most functional proteins that carries variety of endogenous substances like some hormones, fatty acids and exogenous molecules such as drugs (1, 2). Similar to its particular properties like specific secondary structure, stability, water solubility and high binding affinity to different hydrophobic ligands, HSA plays a vital role in homeostasis adjustment (3-6). HSA has 585 amino acids with three domains (I, II and III) and six sub-domains where the binding sites of drugs are located in sub-domains IIA and IIIA according to its crystal structure (7-9). Binding of a drug to its carrier protein has critical role in drug’s distributions, effectiveness and excretion (10). In psychobiology studies, cortisol is well-known as a marker of stress, depression and anxiety that can be measured as a free and total amount in blood. It should be noted that the total form of cortisol correspond free and bounded forms of cortisol. Albumin and corticosteroid binding globulin (CBG) are two cortisol carriers in plasma (11). This steroid hormone has a major role in response to the physiological and mental stress (12). Fluoxetine (N-methyl-c-[4-(trifluoromethyl) phenoxy] benzenepropanamine) (FLX) is one of the drugs that is prescribed for the treatment of mental disorders like stress, depression, panic and obsession (13). FLX has a binding site on the sub-domain IIA that located on the site I of HAS. After its binding, it induces conformational changes in HSA structure (14). Regarding the binding of cortisol and anti-stress drugs like FLX, it seems that binding each of these substances can influence the binding affinity of another one. There are many reported in-vivo studies about the effect of FLX in Rats (15). However; there is not sufficient data about FLX cortisol interference. In this paper, we report a detailed study of the binding effects of cortisol and FLX to HSA in order to determine the mutual relationships.
Experimental
Essentially fatty acid-free human serum albumin was purchased from sigma. Tris buffer 50 mM, pH 7.5 was used for all experiments. Fluoxetine hydrochloride was obtained as a gift sample from Tehran Daru Company and Hydrocortisone was obtained as a free sample from Iran Hormone Company. All of the other materials were of analytical reagent grade and deionized water was used throughout the study.
Subjects
The subjects were 20 experimentally naïve male Wistar rats weighing approximately 300 g at the beginning of the experiment. They were singly housed in 24.0 × 17.5 × 19.0 cm metal cages and were maintained on a 12 h dark-light cycle (lights on at 7:00) in a temperature of 37°C. Half of the subjects were maintained at 80% of their free-feeding body weight, whereas the other half had free access to standard rodent lab food. Water was always available.
Drug administration
To characterize the pharmacokinetic disposition of fluoxetine, a bolus dose of fluoxetine (5 mg/Kg as free base) dissolved in isotonic saline (0.9% NaCl) was administered by tail vein injection into the control and sample Rats. One hour after Post-injection, the whole blood samples were harvested into the borosilicate test tubes by the blood collection of anesthetized animal’s orbital sinus. The whole blood samples were allowed to stand at the room temperature for 1 h and were subsequently centrifuged at 3000 g for 10 min. Harvested serum samples were stored at -20°C. Fluoxetine hydrochloride was dissolved in Tween-80 plus 0.9% NaCl. Daily intraperitoneal injections (IP injections) of 5 mg/Kg were administered for 1 day, at the same time in each group, in the tail-treated subjects. Rats in the acute treatment groups received the same dose but only once.
The corresponding treatment was given to the animals in the control group, which received tail injections (0.9% NaCl). The drug dosage was selected on the basis of its effectiveness on chronic behavioral procedures such as polydipsia (36) and stress-induced anhedonia (37).
Methods
pH meter study
The pH meter studies of incubated HSA in the certain condition were performed by gradually measuring pH-values against the added 50 μL of 0.1 M HCl.
UV-spectroscopy study
The UV-Vis absorption spectra of HSA from 230 nm to 320 nm were obtained by Unico spectrophotometer. HSA was incubated for 5 min in a 50 mM Tris buffer under the applied conditions and then the spectra were taken.
Fluorescence spectroscopy study
The fluorescence spectra of HSA were obtained by Varian Cary Eclipse Fluorescence Spectrophotometer in the condition as like Ultraviolet Spectroscopy (UV-spectroscopy) experiments.
Circular dichroism (CD) study
Circular dichroism (CD) experiments were done on an Aviv 215 spectropolarimeter using a 0.1 cm cell at 0.2 nm intervals and the CD parameters of HSA were calculated and illustrated as a function of wavelength in Far-UV region. Reproducibility of data was proud by repitation. Protein concentrations for pH meter, UV-spectroscopy, fluorescence and CD experiments were 0.7, 0.7, 0.07 and 0.2 mg/mL, respectively. For all experiments, drug and hormone concentrations were 12 mg/L and 4 mg/L, respectively.
Results and Discussion
In many studies, it is reported that the binding of drug and other substances such as some hormones, changes the tertiary structure of HSA (16). In this study, it was proposed that binding cortisol as an important stress hormone and FLX as a well-known anti-stress drug will change the HSA structure. Denaturation is a common process for studying the structural aspects of proteins (17). Several conditions are introduced as protein denaturant such as: temperature, chemical reagents, pH and etc. (18-20). Since the titratable groups in proteins are different (21), the acid denaturation process of proteins may occur dissimilarly. Here, it is proposed that binding FLX and the cortisol effect on the HSA properties and therefore, acid denaturation of HSA in absence and presence of hormone and drug, is investigated (see Figure 1). As shown in Figure 1, the pH meter findings indicate that the HSA denaturations in the presence of drug and hormone are more acid-sensitive than in the absence of the two reagents.
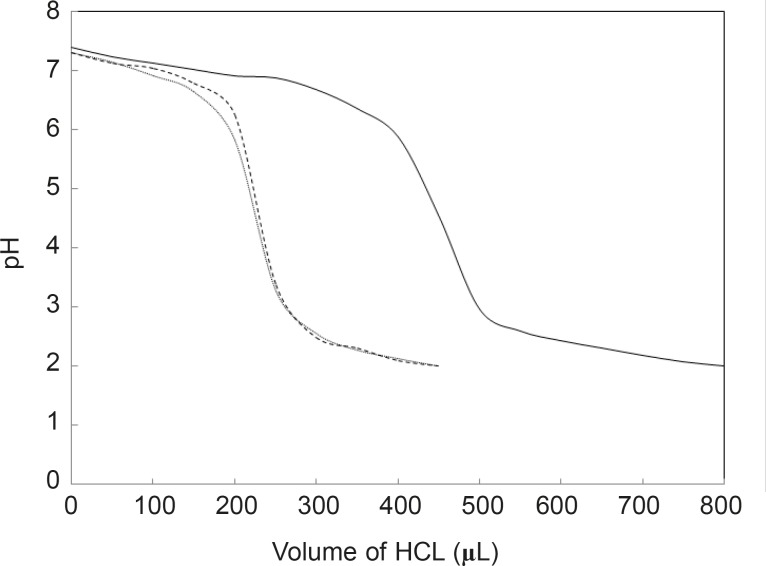
pH meter curves can be classified into two sets; the first set is HSA without any additive and the second includes HSA in the presence of two substances. In spite of contrast functions of hormone and drug in human body (stress generation and anti-stress properties), the very surprisingly point related to the acid denaturation of HSA is the similarity of curves in the presence of two substances. In correspond to the pH meter findings, it can be concluded that FLX may act as a competitive ligand for binding cortisol to HSA. UV Absorption spectroscopy is a method for the study of aromatic groups of proteins; it investigates the accessibility of these groups related to the surface of tertiary structure of protein (22, 23). For a better resolution, the UV spectra of HSA are provided in the same conditions (see Figure 2).
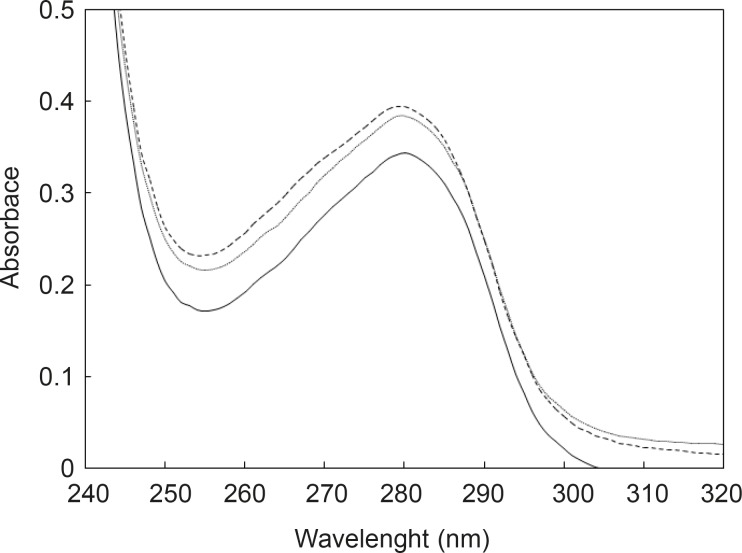
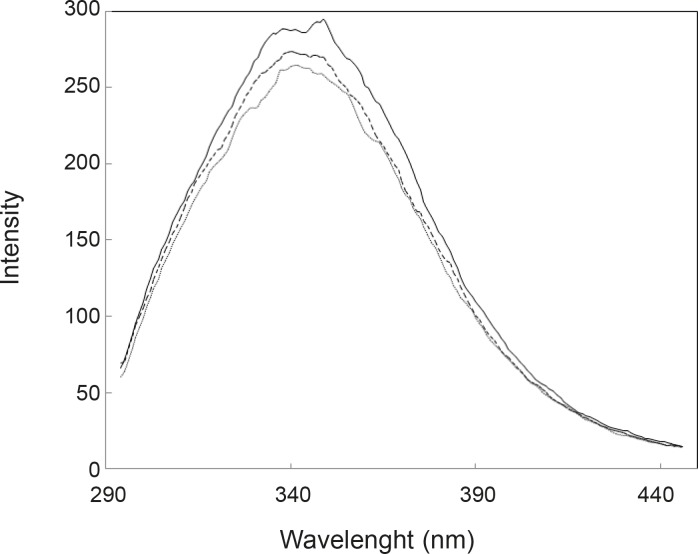
As it is depicted in the Figure 2, UV spectra of HSA in the presence of FLX and cortisol show approximately complete similarity (especially in the 280 nm) and are different with HSA spectrum. The amount of absorption in 280 nm wavelength refers directly to the absorption of aromatic groups (24, 25), therefore, nearly equal hyperchromism of HSA in the presence of FLX and cortisol indicates that these groups are exposed due to the occurrence of conformational change in the protein in similar manner. UV-spectroscopy results confirm the pH meter findings. Fluorescence spectroscopy is a sensitive method for the monitoring of protein tertiary structure change (26). Fluorescence signal (light emission) for a protein refers to the energetic levels of molecular structure (27). The main fluorophore of protein is tryptophan (28). Several conditions can affect the molecular energetic levels and therefore fluorescence properties of a protein (e.g. chemical components), bufferic condition and act (29). Presence of FLX and cortisol cusses a mild reduction of quantum yield of HSA (see Figure 3); it seems that drug and hormone are affected similarly on the fluoroscopy properties of HSA. The fluorescence data in correspond to the UV-spectroscopy and pH meter confirms that cortisol and FLX promote the HSA conformational change in the same direction. CD-spectroscopy is a suitable method for monitoring the secondary and tertiary structure of proteins (30, 31).
Far-Ultraviolet Circular Dichroism (Far-UV CD) spectroscopy is a powerful technique that is used to determine the amount of secondary structures of proteins (32). Variation of CD parameters in 222 nm is related tightly to the rate of α-helix change (33-34). In many studies, it is reported that tertiary structural changes of proteins are accompanied by the secondary structural change of proteins (35). By consider that the two reagents induce conformational change in HSA, it can be expected that HSA may be undergo the secondary structural change. Far-UV CD spectra of HSA in the absence and presence of FLX and cortisol are illustrated in Figure 4.
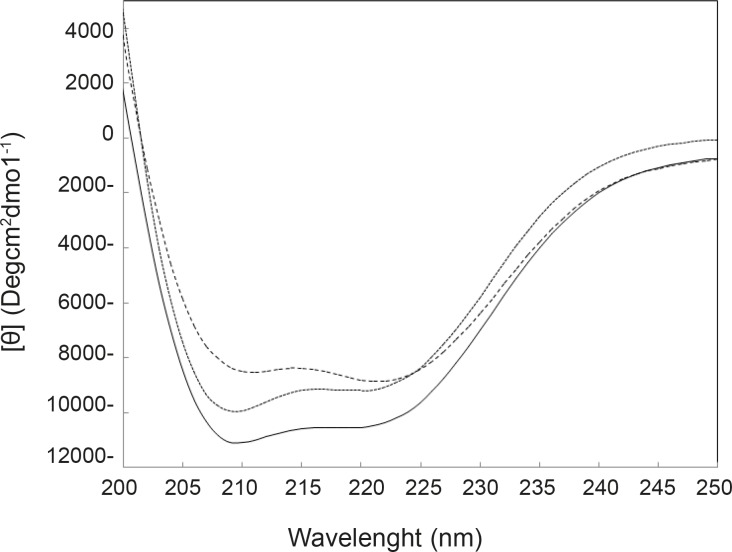
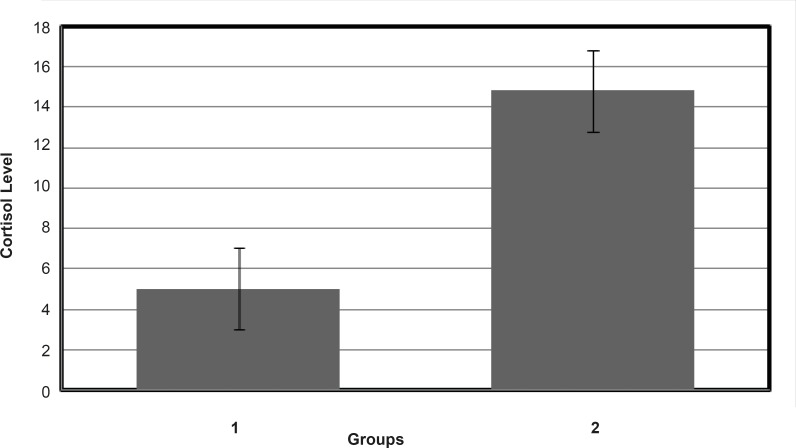
Results of CD experiments (see Figure 4) indicate that HSA in the presence of FLX and cortisol loses a few amounts of its α-helix component in the mild and approximately similar manner. In order to understand the FLX effect on the blood cortisol level, the amounts of blood cortisol were measured 1 h after the tail injection of (0.5 mg/Kg) FLX in Rat. The results are depicted in the Figure 5. As it is shown in Figure 5, cortisol level in the presence of drug is increased from 4.5 to 14.5. Statistical analysis confirms that the presence of FLX effectively alerts blood cortisol level. Finally, it can be concluded that FLX and cortisol alter structural aspects of HSA in human blood in similar manner, so, this findings lead to the reasonable conclusion that FLX is a competitive ligand for binding cortisol-HSA. This conclusion expresses that FLX limits the transports of cortisol in blood and therefore, alters its effects as a stress factor, which is unidirectional with the function of drug.
Acknowledgements
References
-
1.
Muller WE, Wollert U. Human serum albumin as a ‘silent receptor’ for drugs and endogenous substances. Pharmacol. 1979;19:56-59.
-
2.
Hesami Takallu S, Rezaei Tavirani M, Kalantari SH, Amir Bakhtiarivand M, Mahdivi SM. Co-amoxiclav effects on the structual and binding properties of human serum albumin. Iranian J. Pharm. Res. 2010;9:251-257.
-
3.
Zhang L, Wang K, Zhang X. Study of the interactions between fluoroquin-olones and human serum albumin by affinity capillary. Electrophoresis Fluorescence Method Anal. Chim. 2007;603:101-110.
-
4.
Liu M, Zhu L, Qu X, Sun D, Li L, Lin R. Studies on the binding of paeonol and two of its isomers to human serum albumin by using microcalorimetry and circular dichroism. J. Chem. Thermodyn. 2007;39:1565-1570.
-
5.
Ya-Bei H, Shi-Jun W, Yan-Qin Z, Zhang Y, Xiao-Yan G, Ying-Cai T, Da-Ming Z. Investigation on the interaction of prulifloxacin with human serum albumin: a spectroscopic analysis. Chem. Pharm. Bull. 2010;58:582-586. [PubMed ID: 20410649].
-
6.
Lu Y, Cui F, Fan J, Yang Y, Yao X, Li J. Interaction of human serum albumin with N-(4 ethoxyphenyl)-N0-(4-antipyrinyl), thiourea using spectroscopies and molecular modeling method. J. Lumin. 2009;129:734-740.
-
7.
Carter DC, Ho JX. Structure of serum albumin. Adv. Protein Chem. 1994;45:153-203. [PubMed ID: 8154369].
-
9.
Salkowska A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002;614:227-232.
-
10.
Oppenheimer JH. Interactions of drugs with thyroid hormone binding sites. Ann. NY. Acad. Sci. 1973;226:333-340. [PubMed ID: 4203420].
-
11.
Emptoz-Bonneton A, Cousin P, Seguchi K, Avvakumov G, Bully C, Hammond G, Pugeat M. Novel human corticosteroid-binding globulin variant with low cortisol-binding affinity. J. Clin. Endocrinol. Metab. 2000;85:361-367. [PubMed ID: 10634411].
-
12.
Levine A, Zagoory-Sharon O, Feldman R, Lewis J, Weller A. Measuring cortisol in human psychobiological studies. Physiol. Behav. 2007;90:43-53. [PubMed ID: 17055006].
-
13.
Carr RR, Ensom MH. Fluoxetine in the treatment of premenstrual dysphoric disorder. Ann. Pharmacother. 2002;36:713-717. [PubMed ID: 11918525].
-
14.
Katrahalli U, Jaldappagari S, Kalanur S. Probing the binding of fluoxetine hydrochloride to human serum albumin by multispectroscopic techniques. Spectrochimica Acta Part A. 2010;75:314-319.
-
15.
Raap DK, Evans S, Garcia F, Li Q, Muma NA, Wolf WA, Battaglia G, Vandekral D. daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J. Pharmacol. Experiment. Ther. 1999;288:98-106.
-
16.
Huang B, Dass C, Kim H. Probing conformational changes of human serum albumin due to unsaturated fatty acid binding by chemical cross-linking and mass spectrometry. Biochem. J. 2005;387:695-702. [PubMed ID: 15588254].
-
17.
Rezaei-Tavirani M, Moghaddamnia SH, Ranjbar B, Amani M, Marashi SA. Conformational study of human serum albumin in pre-denaturation temperatures by differential scanning calorimetry, circular dichroism and UV Spectroscopy. J. Biochem. Mol. Biol. 2006;39:530-536. [PubMed ID: 17002873].
-
18.
Karantzeni I, Ruiz C, Liu CC, Licata V. Comparative thermal denaturation of Thermus aquaticus and Escherichia coli Type 1 DNA polymerases. Biochem. J. 2003;374:785-792. [PubMed ID: 12786603].
-
19.
Dong A, Kendrick B, Kreilga L, Matsuura J, Manning M, Carpenter JU. Spectroscopic study of secondary structure and thermal denaturation of recombinant human factor XIII in aqueous solution. J. Biochem. Biophys. 1997;347:213-220.
-
20.
Herskovits T, Jallet H, Gadegbeku B. On the structural stability and solvent denaturation of proteins. J. Biol. Chem. 1970;245:4544-4550. [PubMed ID: 5532229].
-
21.
Klingen A, Bombarda E, Ullmann GM. Theoretical investigation of the behavior of titratable groups in proteins. Photochem. Photobiol. Sci. 2006;5:588-596. [PubMed ID: 16761087].
-
22.
Upstone S. Ultraviolet-Visible Light Absorption Spectrophotometry in Clinical Chemistry. John Wiley and Sons Ltd, Chichester. 2000:1699-1714.
-
23.
Lange R, Frank J, Saldana JL, Balny C. Fourth derivative UV-spectroscopy of proteins under high pressure I. Factors affecting the fourth derivative spectrum of the aromatic amino acids. Eur. Biophys. J. 1996;24:277-283.
-
24.
Rzaei-Tavirania M, Moghaddamnia SM, Ranjbar B, Namaki S, Zolfaghari P. The Effects of acetaminophen on human serum albumin (HSA) Iranian J. Pharm. Res. 2005;4:239-244.
-
25.
Tall A, Shiply GG, Small D. Conformational and thermodynamic properties of apo A-l of human plasma high density lipoproteins. J. Biol. Chem. 1976;251:3749-3755. [PubMed ID: 6462].
-
26.
SchmittV, GlangS, Preis J, Detert H. Proton-induced multiple changes of the absorption and fluorescence spectra of amino-aza-oligo(phenylenevinylene)s. Sensor Lett. 2008;6:524-530.
-
27.
Zhong D, Pal SK, Zhang D, Chan SI, Zewail AH. Femtosecond dynamics of rubredoxin: tryptophan solvation and resonance energy transfer in the protein. Proc. Natl. Acad. Sci. USA. 2002;99:13-18. [PubMed ID: 11752400].
-
28.
Szmacinski H, Ray K, Lakowicz JR. Metal-enhanced fluorescence of tryptophan residues in proteins: Application toward label-free bioassays. Anal. Biochem. 2009;38:358-364. [PubMed ID: 19073133].
-
29.
Burshtein EA, Bushueva TL, Permyakov EA. Using fluorescence properties of protein chromophores in studies of equilibrium structural mobility of macromolecules. J. Appl. Spectr. 1978;28:4653-657.
-
30.
Kelly SM, Price N. The use of circular dichroism in the investigation of protein structure and function. Cur. Protein Peptide Sci. 2000;1:349-384.
-
31.
Ranjbar B, Gill P. Circular dichroism techniques: biomolecular and nanostructural analyses- a review. Chem. Biol. Drug Des. 2009;74:101-120. [PubMed ID: 19566697].
-
32.
Navea S, Tauler R, Goormaghtigh E, Juan A. Chemometric tools for classification and elucidation of protein secondary structure from infrared and circular dichroism spectroscopic measurements. Proteins Structure Function Bioinformatics. 2006;63:527-541.
-
33.
Piszczek G, D’auria S, Staiano M, Rossi R, Ginsburg A. Conformational stability and domain coupling in D-glucose/D-galactosebinding protein from Escherichia coli. J. Biochem. 2004;381:97-103.
-
34.
Nair SK, Thomas TJ, Greenfield NJ, Chen A, He H, Thomas T. Conformational dynamics of estrogen receptors α and β as revealed by intrinsic tryptophan fluorescence and circular dichroism. J. Mol. Endocrinol. 2005;35:211-223. [PubMed ID: 16216903].
-
35.
Lee JS, Satoh T, Shinoda H, Samejima T, Wu S, Chiou S. Effect of heat-induced structural perturbation of secondary and tertiary structures on the chaperone activity of a-crystalline. Biochem. Biophys. Res. Commun. 1997;237:277-282. [PubMed ID: 9268700].
-
36.
Woods A, Smith C, Szewczak M, Dunn RW, Cornfeldt M, Corbett R Selective serotonin re-uptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive compulsive disorder. Psychopharmacology. 1993;112:195-198.
-
37.
Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology. 1992;109:433-438. [PubMed ID: 1365858].