Abstract
Keywords
Artemisia annua L. Crude extract Hemostasis In-vitro Plasma Recalcification time
Introduction
Blood loss, while minor in every day cuts and bruises, is one of the main causes of mortality. Hemorrhage threatens the life safety of patients and the wounded in trauma care and surgical intervention. Hemorrhage is the main reason in the causes of death in 48 h after trauma, which accounts for 80% in all trauma accident (1). Early control of hemorrhage remains the most effective strategy for treating combat casualties. Catastrophic blood loss often results in hemorrhagic shock as demonstrated in animal models (2-4), resembling human outcomes (5-7). Therefore development of compounds to improve hemostasis and save patient’s life in the trauma is of medical importance.
A number of hemostatic agents have recently been deployed to the warfront that can be used to arrest bleeding before surgical control of the source (8). The ideal hemostatic agent would be easy to use, inexpensive, and rapidly available with no special storage requirements, non-immunogenic, versatile, and biologically inert with minimal side effects (9). Medicinal plants are used with in a context of a traditional medicine that confronts health and illness from an integral vision.
Artemisia annua L. is the only plant amedica which has been recognized to research and develope as the standards of western medicine research by the WHO in China. It grows wild in Europe and America, which is cropped on a large scale in China, Vietnam, Turkey, Iran, Afghanistan and Australia now (10). It is a famous herbs known for its highest efficiency, most effective and lowest toxicity in treating ague since artemisinin was extracted in 1977. It has been the subject of intensive phytochemical investigation following the discovery of the anti-malarial drug artemisinin (11). At present, most studies focus on artemisinin and its derivatives, while the study and report about the other active components are rare. Effect of Artemisia annua L. on hemostasis is known in traditional medicine (12-15). This paper purposed to further discover new indication Artemisia annua L. Screening the hemostatic active fraction of Artemisia annua L. in-vitro to expand multiple uses of resources of Artemisia annua L.
Experimental
Plant material
Artemisia annua L. was collected from the GAP cultivation base in Youyang, Chongqing, southern China in september 2006 and dried. It was verificatied by researcher Luo Rongchang, and preserved in college of bioengineering, Chongqing University, P.R.China.
Laboratory animal
Japan purely rabbits utilized in the study (male/female 2.5-3.0 kg) purchased from the animal office in Daping, ChongQing, China.
Main instrument and reagent
AKTA Prime Plus bioseparation and purification system; polyamide column; MCI chromatographic column; rotatory evaporator RE-S2; LDZS2 equilibrium hydroextractor; thermostatic waterbath; ethanol; petroleum ether; acetic ether; n-butanol; sodium citrate. All chemicals were of analytical reagent grade and used as received which purchased from East of Sichuan Chemical Industry Group. Yunnan Baiyao purchased from Yunnan Baiyao Group Co., Ltd; doubly distilled water was used throughout.
Blood manipulation
Blood samples, obtained freshly from normal rabbits. It was collected in plastic tubes and anticoagulated with sodium citrate 3.8% (1 part citrate: 9 parts blood) for the recalcification time test. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were immediately prepared according to the standard procedures (16): PRP was obtained by collecting the supernatant plasma after the blood samples were centrifuged at 1000 rpm for 10 min, and PPP was obtained after the blood samples were centrifuged at 3000 rpm for 20 min. The plasma recalcification time test was performed soon after PRP and PPP were obtained.
Measurement of hemostatic activity
The potential of plant extracts to hemostasis was assessed based on a procedure described by Kang Chen et al. (17). In the determination of the recalcification time of blood plasma, 0.1 mL of the plasma (PPP) was transferred to the detection extraction. The mixture was incubated at 37°C for 1 min, then recalcified by addition of 0.1 mL M/40 CaCl2 warmed to 37°C. A stopwatch was start as the calcium salt was added. The end of solidification reached since the plasma is fully concreting.
We gain the shortening rate of cloting time through the next mathematical formula:
(Equation 1)
(R = shortening rate of clotting time; T1 = clotting time of control group; T2 = clotting time of medication administration team).
Purification of hemostatic active fraction in Artemisia annua L.
A quantity (40 kg) of Artemisia annua L. was extracted in a diffusion apparatus with 75% ethanol. The ethanolic extracts were filtered and evaporated to dryness under reduced pressure in a rotary evaporator (18).
We recorded the crude extract as O, and then extracted the crude extract by equal volume of petroleum ether (recorded as: A), ethyl acetate (recorded as: B), n-butanol (recorded as: C) one by one, the remaining part after the extraction of n-butanol (recorded as: D). The extracts then transferred to vials, kept at 4°C and examined for PRT.
The extract of n-butanol was purificated by polyamide. Water eluting first, which was recorded as C1, and followed by different concentrations of ethanol(30%, 50%, 70%, 95%) to elute, which were record as C2, C3, C4, C5 respectively.
C1 was segregated by column chromatography of MCI gel to gradient elute. The eluting solvent were water (recorded as: C11), and then eluted by 20% methanol, 60% methanol (recorded as: C12, C13 respectively). The conditions of the separation were: wave 365 nm; flow rate: 2 mL/ min; fraction size: 15 mL; pressure: 0.3 MPa.
The preparation of physic liquor: 0.25 g extracts were dissolved in 50 mL doubly distilled water, and make sure it to be 0.5% concentration, then we filtering it by disposable filter.
Data analysis
The potency of artemisia annua L. in stopping bleeding and cloting retraction was indicated as r-value (the shortening rate of clotting time to the negative control). The positive control is Yunnan BaiYao, which is the most widely used hemostatic agent in China. Repeat 6 times, significance analysis and statistical comparisons were performed by means of the originpro7.5.
Results
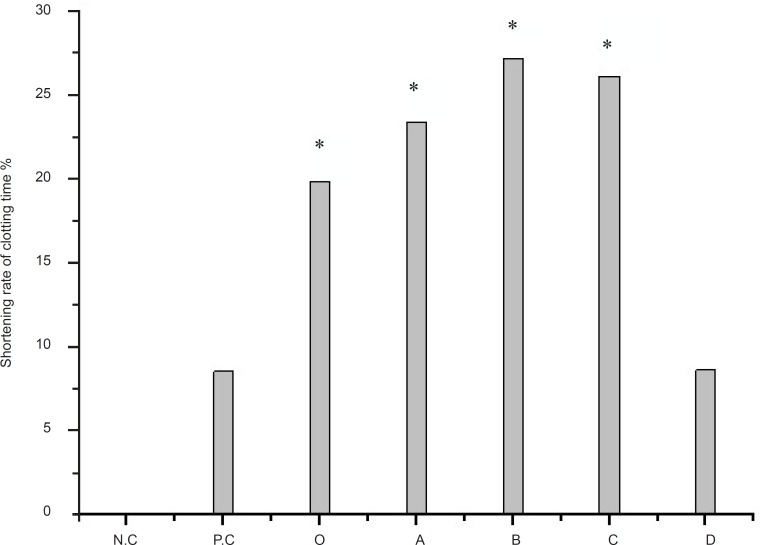
The hemostatic activity of the crude extract and the four extracts with organic solvents of Artemisia annua L. were provided in Figure 1. As displayed in Figure 1, the crude extract of Artemisia annua L. has the hemostatic activity, and the r-value is 19.85%, while the r-value of positive control is 8.54%. In Figure 1, because the ethyl acetate extract is not completely dissolved and there is granular which can accelerate the solidification of the plasma in the physic liquor, the r-value of ethyl acetate extract is a little high than the n-butanol extract. We also did another test called as clotting time adopting coagulation plate (ACP) (19), and the result showed that the r-value of n-butanol extract is higher. There was significant effect of the crude extract and n-butanol extract on PRT.
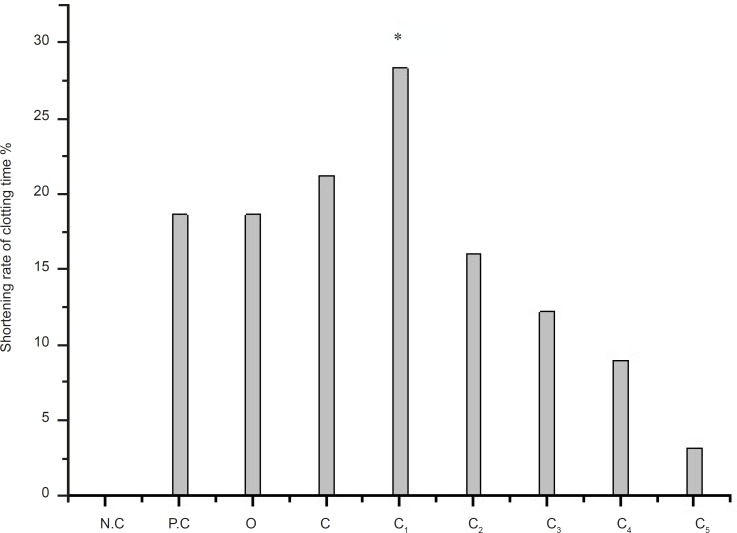
The effect of different eluting solvents from C (n-butanol extract) on PPT is shown in Figure 2. C1 has the higher r-value than the C. It is significant effect fraction after the n-butanol extract. The r-value is 28.36%, while the r-value of C is 21.27% and the r-value of positive control (recorded as: P.C ) is 18.68%.
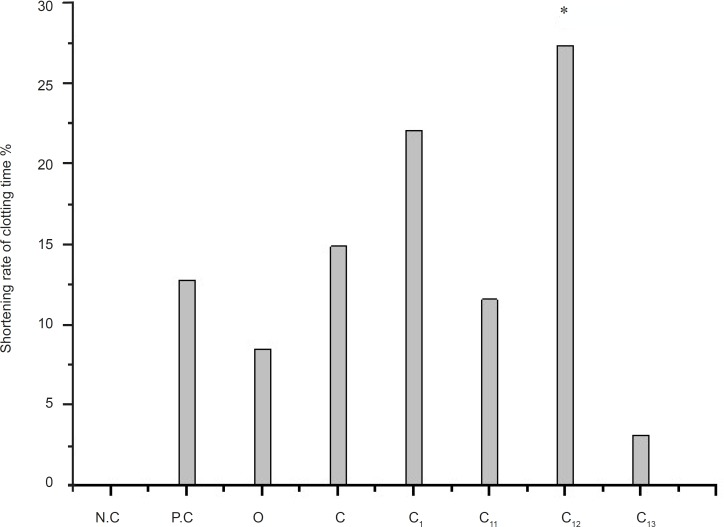
The effect of different eluting solvents from C1 (n-butanol extract) on PPT is shown in Figure 3. The r-value are followed by: O (8.51%); C (14.89%); C1(22.11%); C12(27.37%); P.C (12.77%). The rabbits we used in the research are different in every experiment, so the r-value of same part is slightly different in the three figures, while we determined the PRT of crude extract and the source of the activity site in every experiment. So the results are scientific and rationality.
Discussion
Hemostasis is important to save patient’s life. Plasma recovery-calcium time: decoagulant combined with the calcium of plasma to block up the coagulation process. The result shows that the Artemisia annua L. extract and C12 have obvious pro-coagulant effect in-vitro. The C12 from Artemisia annua L. might be the effective fraction. C12 is the part of 20% methanol fraction after column chromatography of MCI gel is the hemostatic active fraction of Artemisia annua L. The shorten rate of clotting time is (27.37%).
Controlling hemorrhage will always remain a top priority in trauma care, and the development of materials to achieve this goal more effectively is of obvious benefit. In response to the changing combat and trauma casualty care, there has been an increase in efforts to develop better hemostatic agents. An ideal agent should be effective, easy to use, safe, logistically superior, and durable.
With the current therapeutic condition of hemorrhage, an effective hemostatic drug is significant. Because Artemisia annua L. is sufficient, and the price is cheaper, screening the hemostatic active fraction of Artemisia annua L. to develop haemostat is superiority. Since Artemisia annua L. is derived from a plant source that the risk of disease transmission or allergic reaction is minimal. This also provides a readily available source to keep the overall cost of the product low.
The present findings, although requiring confirmation by a larger trial, show that further research is needed with longer survival studies and direct comparisons to other hemostatic agents before widespread acceptance is prudent.
Acknowledgements
References
-
1.
Sui J, Wang BC, Yu ZW. A novel hemostatic model with triple protective functions. Med. Hypotheses. 2009;72:186-187. [PubMed ID: 18851898].
-
2.
Vallejo JG, Nemoto S, Ishiyama M, Yu B, Knuefermann P, Diwan A, Baker JS, Defreitas G, Tweardy DJ, Mann DL. Functional significance of inflammatory mediators in a murine model of resuscitated hemorrhagic shock. Am. J. Heart Circ. Physiol. 2005;288:H1272-H1277.
-
3.
Zakaria ER, Garrison N, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J. Trauma Inj. Infect. Crit. Care. 2005;58:408-499.
-
4.
Toth B, Yokoyama Y, Schwacha MG, George RL, Rue LW, Bland KI, Chaudry IH. Insights into the role of interleukin-6 in the induction of hepatic after trauma-hemorrhagic shock. J. Appl. Physiol. 2004;97:2184-2189. [PubMed ID: 15298985].
-
5.
Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches. Int. J. Mol. Med. 1999;4:575-583. [PubMed ID: 10567665].
-
6.
Geppert A, Steiner A, Zorn G, Delle-Karth G, Koreny M, Haumer M, Siostrzonek P, Huber K, Heinz G. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit. Care Med. 2002;30:1987-1994. [PubMed ID: 12352031].
-
7.
Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417-422. [PubMed ID: 9872681].
-
8.
Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Mil. Med. 2005;170:63-69. [PubMed ID: 15724857].
-
9.
Humphreys MR, Castle EP, Andrews PE, Gettman MT, Ereth MH. Microporous polysaccharide hemospheres for management of laparoscopic trocar injury to the spleen. Am. J. Surg. 2008;195:99-103. [PubMed ID: 18070734].
-
10.
Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Curr. Sci. India. 2001;80:35-48.
-
11.
Sy LK, Brown GD. Three sesquiterpenes from Artemisia annua. Phytochem. 1998;48:1207-1211.
-
12.
Feng WY. Preliminary exploration of Artemisia annua L. and its praeparatum. Tibetan Med. J. 1978;2:82-83.
-
13.
Duan JP. Healing epistaxis through crystal sugar soup of Artemisia annua L. New J. Trad. Chinese Med. 2005;37:31.
-
14.
Haghi G, Safaei A, Safaei Ghomi J. Identification and determination of flavonoids in leaf, dried aqueous and dried hydroalcoholic extract of Artemisia absinthium by HPLC. Iranian J. Pharm.Res. 2004;Suppl 2:89-90.
-
15.
Li YF. Healling upper gastrointestinal bleeding with the expulsion of heat. Chinese Med. Emerg. Case. 2000;6:244.
-
16.
Ye YW, Li JZ, Wang YS. Clinic Detection and Diagnosis. Beijing: People’s Hygiene Publishing House; 1989. 1116 p.
-
17.
Chen K, Liu DZ, Nie LH, Yao SZ. Behaviour of surface acoustic wave interdigitated array electrode sensor in non-aqueous solution and determination of blood plasma recalcification time. Biosens. Bioelectton. 1996;11:515-521.
-
18.
Souri E, Amin G, Dehmobed-Sharifabadi A, Nazifi A, Farsam H. Antioxidative activity of sixty plants from Iran. Iraian J. Pharm. Res. 2004;3:55-59.
-
19.
Wang XQ, Luo XD, Wu SH. Hemostatic properties of chloroform-water phase extract from a Chinese herb. J. Clin. Rehabilitative Tissue Eng. Res. 2007;24:4722-4725.