Abstract
Background and Objective: There is a paucity of data regarding tolerability of alkaline drugs administered subcutaneously. The aim of this study was to assess the tolerability of alkaline preparations of human albumin delivered subcutaneously to healthy humans.
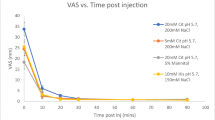
Methods: We compared the tolerability of neutral versus alkaline (pH 10) formulations of human albumin in ten volunteers. With an intent to minimize the time required to reach physiological pH after injection, the alkaline formulation was buffered with a low concentration of glycine (20 mmol/L). Each formulation was given at two rates: over 5 seconds and over 60 seconds. A six-point scale was used to assess discomfort.
Results: For slow injections, there was a significant difference between pH 7.4 and pH 10 injections (0.4±0.2 vs 1.1±0.2, mean±SEM; p = 0.025), though the degree of discomfort at pH 10 injections was only ‘mild or slight’. For fast injections, the difference between neutral and alkaline formulations was of borderline significance. Inflammation and oedema, as judged by a physician, were very minimal for all injections, irrespective of pH.
Conclusion: For subcutaneous drug administration (especially when delivered slowly), there was more discomfort associated with alkaline versus neutral formulations of albumin, though the discomfort was mild. This study suggests that there is little discomfort and inflammation resulting from subcutaneous administration of protein drugs formulated with weak buffers at alkaline pH.






Similar content being viewed by others
References
Goenaga M, Millet M, Sanchez E, et al. Subcutaneous furosemide [letter]. Ann Pharmacother 2004; 38: 1751
Verma AK, da Silva JH, Kuhl DR. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother 2004; 38: 544–9
Ward WK, Massoud RG, Szybala CJ, et al. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol 2010; 4: 1311–21
Brazeau GA, Cooper B, Svetic KA, et al. Current perspectives on pain upon injection of drugs. J Pharm Sci 1998; 87: 667–77
Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exper Therap 1944; 83: 377–90
Klement W, Arndt JO. Pain on i.v. injection of some anaesthetic agents is evoked by the unphysiological osmolality or pH of their formulations. Br J Anaesth 1991; 66: 189–95
Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010; 33: 1282–7
El-Khatib FH, Russell SJ, Nathan DM, et al. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010; 2: 27ra27
Fransson J, Espander-Jansson A. Local tolerance of subcutaneous injections. J Pharm Pharmacol 1996; 48: 1012–5
Acknowledgements
We are grateful to the research subjects and to the Juvenile Diabetes Research Foundation and the Legacy Good Samaritan Foundation for financial support. With regard to potential conflicts of interest, a patent has been submitted by the authors for the use of glucagon formulated at alkaline pH in persons with diabetes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ward, W.K., Castle, J.R., Branigan, D.L. et al. Discomfort from an Alkaline Formulation Delivered Subcutaneously in Humans. Clin Drug Investig 32, 433–438 (2012). https://doi.org/10.2165/11632840-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11632840-000000000-00000