Abstract
Background: Patients with epilepsy, including children, have an increased risk of mortality compared with the general population. Antiepileptic drugs (AEDs) were the most frequent class of drugs reported in a study looking at fatal suspected adverse drug reactions in children in the UK.
Objective: The objective of the study was to identify cases and causes of death in a paediatric patient cohort prescribed AEDs with an associated epilepsy diagnosis.
Methods: This was a retrospective cohort study supplemented with general practitioner-completed questionnaires, post-mortem reports and death certificates. The setting was UK primary care practices contributing to the General Practice Research Database. Participants were children and adolescents aged 0–18 years prescribed AEDs between 1993 and 2005. Causality assessment was undertaken by a consensus panel comprising paediatric specialists in neuropathology, neurology, neuropsychiatry, paediatric epilepsy, pharmacoepidemiology and pharmacy to determine crude mortality rate (CMR) and standardized mortality ratios (SMRs), and the likelihood of an association between AED(s) and the event of death.
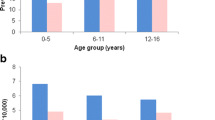
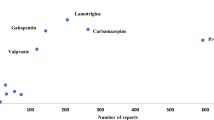
Results: There were 6190 subjects in the cohort (contributing 26 890 person-years of data), of whom 151 died. Median age at death was 8.0 years. CMR was 56.2 per 10000 person-years and the SMR was 22.4 (95% CI 18.9, 26.2). The majority of deceased subjects had severe underlying disorders. Death was attributable to epilepsy in 18 subjects; in 9 the cause of death was sudden unexpected death in epilepsy (SUDEP) [3.3 per 10 000 person-years (95% CI 1.5, 6.4)]. AEDs were probably (n = 2) or possibly (n = 3) associated causally with death in five subjects. Two status epilepticus deaths were associated causally with AED withdrawal.
Conclusions: Children prescribed AEDs have an increased risk of mortality relative to the general population. Most of the deaths were in children with serious underlying disorders. A small number of SUDEP cases were identified. AEDs are not a major cause of death but in a small proportion of cases, a causal relationship between death and AEDs could not be excluded.






Similar content being viewed by others
References
Appleton RE. Mortality in paediatric epilepsy. Arch Dis Child 2003; 88: 1091–4
Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child 2002; 87: 462–7
Ackers R, Murray M, Besag F, et al. Prioritizing children’s medicines for research: a pharmacoepidemiological study on newer antiepileptic drugs. Br J Clin Pharmacol 2007; 63(6): 689–97
Hollowell J. The general practice research database: quality of morbidity data. Popul Trends 1997; 87: 36–40
Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol 2005; 59(6): 750–5
Rani F, Murray ML, Byrne PJ, et al. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics 2008; 121: 1002–9
McCarthy S, Asherson P, Coghill D, et al. Trends in prescribing prevalence and cessation of ADHD drug treatment among adolescents and young adults in the United Kingdom. Br J Psychiatry 2009; 194(3): 273–7
Thompson PL, Gilbert R, Long PF, et al. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the UK General Practice Research Database. Pediatrics 2009; 123: 424–30
Derby LE, Tennis P, Jick H. Sudden unexplained death among subjects with refractory epilepsy. Epilepsia 1996; 37(10): 931–5
McCarthy S, Cranswick N, Potts L, et al. Mortality associated with attention deficit hyperactivity disorder (ADHD) drug treatment. Drug Saf 2009; 32(11): 1089–96
European Medicines Agency. ICH Topic E11. Clinical investigation of medicinal products in the paediatric population. 2001 Jan. CPMP/ICH/2711/99 [online]. Available from URL: http://www.emea.europa.eu/pdfs/human/ich/271199en.pdf [Accessed 2008 Jun 19]
National Institute for Health and Clinical Excellence (NICE 2004). Newer drugs for epilepsy in children. Technology appraisal 79, 2004 Apr [online]. Available from URL: http://guidance.nice.org.uk/TA79/guidance/pdf/English [Accessed 2008 Jun 19]
World Health Organisation. International statistical classification of diseases and related health problems. 10th rev. Version for 2007 [online]. Available from URL: http://www.who.int/classifications/apps/icd/icd10online/ [Accessed 2008 Jun 19]
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–9
Hanna NJ, Black M, Sander JWS, et al. The national sentinel clinical audit of epilepsy-related death: epilepsy-death in the shadows. London: The Stationery Office, 2002
Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997; 38: S6–8
Bland M. An introduction to medical statistics. London: Oxford University Press, 1993
Berg AT, Shinnar S, Testa FM, et al. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med 2004; 158: 1147–52
Callenbach PM, Westendorp RG, Geerts AT, et al. Mortality risk in children with epilepsy: the Dutch Study of Epilepsy in Childhood. Pediatrics 2001; 107(6): 1259–63
Harvey AS, Nolan T, Carlin JB. Community-based study of mortality in children with epilepsy. Epilepsia 1993; 34(4): 597–603
Sillanpaa M, Jalava M, Kaleva O, et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med 1998; 338(24): 1715–23
Werlin SL, Fish DL. The spectrum of valproic acid-associated pancreatitis. Pediatrics 2006; 118(4): 1660–3
Chapman SA, Wacksman GP, Patterson BD. Pancreatitis associated with valproic acid: a review of the literature. Pharmacotherapy 2001; 21(12): 1549–60
Asconape JJ, Penry JK, Dreifuss FE, et al. Valproate associated pancreatitis. Epilepsia 1993; 34(1): 177–83
Bell GS, Gaitatzis A, Johnson AL, et al. Predictive value of death certification in the case ascertainment of epilepsy. J Neurol Neurosurg Psychiatry 2004; 75(12): 1756–8
Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet 2002; 359: 1891–5
Nashef L, Fish DR, Garner S, et al. Sudden death in epilepsy: a study of incidence in a young cohort with epilepsy and learning difficulty. Epilepsia 1995; 36(12): 1187–94
Stokes T, Shaw EJ, Juarez-Garcia A, et al. Clinical guidelines and evidence review for the epilepsies: diagnosis and management in adults and children in primary and secondary care. London: Royal College of General Practitioners, 2004
Brorson LO, Wranne L. Long-term prognosis in childhood epilepsy: survival and seizure prognosis. Epilepsia 1987; 28(4): 324–30
Donner EJ, Smith CR, Snead OC. Sudden unexplained death in children with epilepsy. Neurology 2001; 57: 430–4
British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatricians and Child Health. British national formulary for children (BNFC). London: BMJ Publishing Group Ltd, RPS Publishing, RCPCH Publications Ltd, 2008
Wong ICK, Lhatoo SD. Adverse reactions to new anti-convulsant drugs. Drug Saf 2000; 23(1): 35–56
Wong ICK, Mawer GE, Sander JWAS. Vigabatrin and visual field constriction. BMJ 1997; 314: 1693–4
Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005 12; 64(7): 1131–3
Acknowledgements
We sincerely thank the following members of the study steering committee: Kathleen and Kevin Bramley (parents of a child with epilepsy), Jane Hanna (Director of Epilepsy Bereaved), Dr Henry Smithson (GP, Former Chair of the Epilepsies Guidelines Group at the National Institute for Health and Clinical Excellence [NICE]) and Dr Lynda Wilton (Pharmacoepidemiologist). We are also grateful to Professor Tim Cole at the Institute for Child Health, London, for his statistical advice, the GPRD verification team and staff at the Office for National Statistics for providing the additional data for this study, and to all the GPs who contributed data to the GPRD.
Contributors: Ian C.K. Wong, Frank M.C. Besag, Ruth Ackers and Macey L. Murray conceived the idea of the study. All authors were involved in the study design. Ruth Ackers and Ian C.K. Wong analysed the data, and Ruth Ackers, Frank M.C. Besag, Elaine Hughes, Waney Squier and Ian C.K. Wong interpreted the data. All authors had full access to the study data and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors drafted, revised and approved the final manuscript. Ian C.K. Wong and Frank M.C. Besag supervised the study. Ian C.K. Wong is the guarantor.
Competing interest statement: Ian C.K. Wong is a member of the NICE Epilepsy Guideline Group, and has received funding from various pharmaceutical companies, including GlaxoSmithKline, Janssen-Cilag, Pfizer and Therakind (manufacturers of lamotrigine, topiramate, gabapentin and midazolam, respectively); however, none of the funding is related to this study. Frank M.C. Besag has received lecture fees, consultancy fees, research grants and equipment grants from and has been sponsored to conferences by various pharmaceutical companies. He was previously Editor-in-Chief of a journal sponsored by GlaxoSmithKline. None of these monies have been paid directly to him; all monies since 2001 paid to NHS Trust. No monies are currently being received from pharmaceutical companies, nor from any source other than his employer, the NHS in the UK. Frank M.C. Besag has recently been sponsored to attend international epilepsy conferences by Eisai, the company that markets rufinamide in the UK. In addition, he is shortly to receive an unrestricted educational grant from Janssen-Cilag to hold a non-profit educational conference at the Royal College of Physicians on the Use of Psychotropic Drugs in Child and Adolescent Psychiatry. Elaine Hughes has received payment for teaching at educational meetings supported by Eisai, UCB Pharma and Janssen-Cilag. Ruth Ackers, Waney Squier and Macey L. Murray have no competing interests.
Funding: The protocol and methodology had also been peer reviewed and approved by the MHRA as the funder of this project. The MHRA reviewed and commented on the results and final report; however, they had no control of the conduct of the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ackers, R., Besag, F.M., Hughes, E. et al. Mortality Rates and Causes of Death in Children with Epilepsy Prescribed Antiepileptic Drugs. Drug-Safety 34, 403–413 (2011). https://doi.org/10.2165/11588480-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11588480-000000000-00000