Abstract
Migraine is a disabling, painful primary headache disorder that is associated with various combinations of neurological, gastrointestinal, autonomic and pain symptoms. Gastrointestinal disturbances associated with migraine, including nausea and vomiting, affect a majority of migraineurs and often result in a delay in taking or avoidance of pharmacological intervention. Gastric stasis and vomiting may lead to delayed or inconsistent absorption of orally administered medications. Many migraineurs awake early in the morning with their attack progressing and already associated with nausea and vomiting. As a result, there is a need for a novel, non-invasive, non-oral delivery system for fast and effective acute treatment of migraine.
There are two non-oral delivery systems currently available in the US for the acute treatment of migraine: three nasal sprays and two injectable formulations. Although nasal sprays depend partially on nasal mucosal absorption, a significant amount of drug is swallowed, transits the stomach and is absorbed in the small intestine, which is not as rapid or effective a route of delivery for those migraineurs with gastric stasis. Sumatriptan is rapidly absorbed by subcutaneous injection with or without a needle, but the invasiveness and discomfort of the delivery, the high incidence of adverse events and the high recurrence rate all limit its use for many patients. Iontophoretic delivery of medication is a non-invasive transdermal approach that uses small amounts of electrical current to promote rapid movement of the ionized drug through the skin and into the systemic circulation. This delivery bypasses hepatic first-pass metabolism and also avoids gastric transit delay and slowing of small intestinal absorption associated with gastrointestinal stasis in migraineurs.
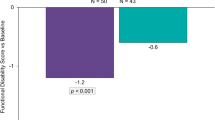
Two pharmacokinetic studies have demonstrated that iontophoretic transdermal delivery of sumatriptan results in rapid and consistent achievement of therapeutic plasma concentrations. These studies also suggest that, by avoiding patient exposure to a rapid rise in and high plasma concentrations of sumatriptan as seen with injectable sumatriptan, transdermal delivery using iontophoresis may significantly reduce typical triptan-related adverse events. A large, randomized, double-blind, placebo-controlled, multicentre clinical trial showed statistically significant efficacy, good tolerability and virtually no triptan-related adverse events.
Iontophoretic delivery of sumatriptan, with a novel transdermal patch device, offers patients a migraine-specific medication that is non-invasive and non-oral. Clinically, transdermal delivery provides rapid and effective relief of migraine while bypassing the gastrointestinal tract, with minimal classic triptan-related adverse effects. This unique approach facilitates the rapid absorption of this migraine-specific triptan, which should improve the chances of consistently achieving a therapeutic plasma concentration of sumatriptan, resulting in effective migraine relief.








Similar content being viewed by others
References
Goadsby PT, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med 2002; 346: 257–70
Menken M. The ambulatory workload of office-based neurologists: implications of the National Ambulatory Medical Care Survey. Arch Neurol 1996; 53(4): 379–81
Sempere AP, Mola S, Medrano V, et al. Descriptive epidemiology of ambulatory neurological care in the Vega Baja (Alicante) area. Rev Neurol 2002; 35: 822–6
Rajput AH, Uitti RJ, Rajput AH. Neurological disorders and services in Saskatchewan: a report based on provincial health care records. Neuroepidemiology 1988; 7: 145–51
Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine II Study. Headache 2001; 41: 646–57
Menken M, Munsat TL, Toole JF. The global burden of disease study: implications for neurology. Arch Neurol 2000; 27: 418–20
Silberstein S, Loder E, Diamond S, et al. Probable migraine in the United States: results of the American Migraine Prevalence and Prevention (AMPP) study. Cephalalgia 2007; 27(3): 220–34
Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia 2004; 24 Suppl. 1: 9–160
Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008; 48(4): 553–63
Hawkins K, Wang S, Rupnow MF. Indirect cost burden of migraine in the United States. J Occup Environ Med 2007; 49(4): 368–74
Silberstein SD. Migraine symptoms: results of a survey of self-reported migraineurs. Headache 1995; 35: 387–96
Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Int Med 1999; 159: 813–8
Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia 2006; 26: 548–53
Ho TW, Mannix LK, Fan X, et al., MK-0974 Protocol 004 study group. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 2008 15; 70(16): 1304–12
Headache Classification Committee, Olesen J, Bousser MG, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006; 26: 742–6
Graham JR, Wolff HG. Mechanisms of migraine headache and action of ergotamine tartrate. Arch Neurol Psychiatry 1938; 39: 737–63
Doenicke A, Brand J, Perrin VL. Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraine. Lancet 1988; 1(8598): 1309–11
Humphrey PPA. How it started. Cephalalgia 2001; 21: 2–5
Tepper SJ, Millson D. Safety profile of the triptans. Expert Opin Drug Saf 2003; 2(2): 123–32
Fuseau E, Petricoul O, Moore KHP, et al. Clinical pharmacokinetics of intranasal sumatriptan. Clin Pharmacokinet 2002; 41: 801–11
Uemura N, Onishi T, Mitaniyama A, et al. Bioequivalence and rapid absorption of zolmitriptan nasal spray compared with oral tablets in healthy Japanese subjects. Clin Drug Investig 2005; 25(3): 199–208
Rapoport AM, Tepper SJ, Bigal ME, et al. The triptan formulations: how to match patients and products. CNS Drugs 2003; 17(6): 431–47
Rapoport AM, Bigal ME, Tepper SJ, et al. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs 2004; 18(10): 671–85
MacGregor EA, Brandes J, Eikermann A. Migraine prevalence and treatment patterns: the global Migraine and Zolmitriptan Evaluation survey. Headache 2003; 43: 19–26
Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache 1999; 39: S20–6
Dahlof C, Aurora SK, Sheftell FD, et al. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden [abstract]. Neurology 2005; 64 Suppl. 1: A336
Aurora SK, Kori S, Barrodale P, et al. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache 2006; 46: 57–63
Volans GN. The effect of metoclopramide on the absorption of effervescent aspirin in migraine. Br J Clin Pharmacol 1975; 2: 57–63
Tokola RA. The effect of metoclopramide and prochlor-perazine on the absorption of effervescent paracetamol in migraine. Cephalalgia 1988; 8: 139–47
Tokola RA, Kangasniemi P, Neuvonen PJ, et al. Tolfenamic acid, metoclopramide, caffeine and their combinations in the treatment of migraine attacks. Cephalalgia 1984; 4:253–63
Prescott LF. Gastric emptying and drug absorption. Br J Clin Pharmacol 1974; 1: 189–90
Volans GN. Migraine and drug absorption. Clin Pharmacokinet 1978; 3: 313–8
Mosek A, Novak V, Opfer-Gehrking TL, et al. Autonomic dysfunction in migraineurs. Headache 1999; 39: 108–17
Avnon Y, Nitzan M, Sprecher E, et al. Autonomic asymmetry in migraine: augmented parasympathetic activation in left unilateral migraineurs. Brain 2004; 127 (Pt 9): 2099–108
Peroutka SJ. Migraine: a chronic sympathetic nervous system disorder. Headache 2004; 44: 53–64
Shechter A, Stewart WF, Silberstein SD, et al. Migraine and autonomic nervous system function: a population-based, case-control study. Neurology 2002; 58: 422–7
Aurora S, Kori S, Barrodale P, et al. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache 2007; 47: 1443–6
Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004; 55(1): 19–26
Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005; 161(6–7): 658–60
Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 2007; 297(13): 1443–54
GlaxoSmithKline. Treximet™ (sumatriptan and naproxen sodium tablets): prescribing information [online]. Available from URL: http://us.gsk.com/products/assets/us_treximet.pdf [Accessed 2008 Jul 7]
Pierce MW. Headache relief: drug discovery and development, March 1, 2008 [online]. Available from URL: http://www.dddmag.com/Article-Anti-migraine-Drugs-Feature-New-Delivery-Methods.aspx [Accessed 2008 Jul 7]
Siegel SJ, O’Neill C, Dube LM, et al. A unique iontophoretic patch for optimal transdermal delivery of sumatriptan succinate [abstract no. F06]. Headache 2007; 47(5): 753
Pierce M, Marbury T, O’Neill C, et al. Zelrix™: a novel transdermal formulation of sumatriptan. Headache 2009 Jun;49(6): 817–25
Goldstein J, Pugach N, Smith T, et al. Acute anti-migraine efficacy and tolerability of Zelrix, a novel iontophoretic transdermal patch of sumatriptan. Research abstract presented at 14th International Headache Congress; 2009 Sep 10–13; Philadelphia (PA). Headache 2010; 50(3): 509–15
Acknowledgements
Funding for this project was provided by NuPathe. Drs Rapoport, Freitag and Pearlman have received consulting honoraria from NuPathe (manufacturers of Zelrix).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rapoport, A.M., Freitag, F. & Pearlman, S.H. Innovative Delivery Systems for Migraine. CNS Drugs 24, 929–940 (2010). https://doi.org/10.2165/11317540-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11317540-000000000-00000