Abstract
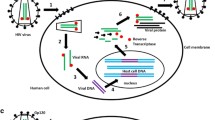
HIV-1 infection is the cause of acquired immune deficiency syndrome (AIDS). Highly active anti-retroviral therapy (HAART) has been successful in reducing the rate of progression to AIDS, but a cure has not yet been achieved. New tools are required to delay progression of infection or to block the replication cycle of HIV. RNA interference (RNAi) has the potential to work as a powerful tool against HIV infection. The mode of action of small interfering RNAs (siRNAs) against their target genes is through sequence complementarity, which in turn results in target degradation. siRNAs are showing enormous potential to be used as a therapeutic tool in various diseases; however, this technology still requires refinement before its full potential can be utilized for the development of HIV therapies.
Similar content being viewed by others
References
Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999 May; 5(5): 512–7
Saag MS, Kilby JM. HIV-1 and HAART: a time to cure, a time to kill. Nat Med 1999 Jun; 5(6): 609–11
Voss G, Villinger F. Adjuvanted vaccine strategies and live vector approaches for the prevention of AIDS. AIDS 2000; 14Suppl. 3: S153–65
Reyes-Darias JA, Sanchez-Luque FJ, Berzal-Herranz A. Inhibition of HIV-1 replication by RNA-based strategies. Curr HIV Res 2008 Nov; 6(6): 500–14
Scherer L, Rossi JJ, Weinberg MS. Progress and prospects: RNA-based therapies for treatment of HIV infection. Gene Ther 2007 Jul; 14(14): 1057–64
Morris KV, Rossi JJ. Lentivirus-mediated RNA interference therapy for human immunodeficiency virus type 1 infection. Hum Gene Ther 2006 May; 17(5): 479–86
Rossi JJ. Expression strategies for short hairpin RNA interference triggers. Hum Gene Ther 2008 Apr; 19(4): 313–7
van Rij RP, Andino R. The silent treatment: RNAi as a defense against virus infection in mammals. Trends Biotechnol 2006 Apr; 24(4): 186–93
Zhu P, Winkler H, Chertova E, et al. 2008 Cryoelectron tomography of HIV-1 envelope spikes: further evidence for tripod-like legs. PLoS Pathog 2008 Nov; 4(11): e1000203
Sougrat R, Bartesaghi A, Lifson JD, et al. 2008 Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog 2007 May 4; 3(5): e63
Dalgleish AG, Beverley PC, Clapham PR, et al. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 1984 Dec 20–1985 Jan 2; 312(5996): 763–7
Klatzmann D, Champagne E, Chamaret S, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 1984 Dec 20–1985 Jan 2; 312(5996): 767–8
Bour S, Geleziunas R, Wainberg MA. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Microbiol Rev 1995 Mar; 59(1): 63–93
Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996 Jun 20; 381(6584): 661–6
Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 1996 Jun 28; 85(7): 1149–58
Feng Y, Broder CC, Kennedy PE, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996 May 10; 272(5263): 872–7
Deng HK, Unutmaz D, KewalRamani VN, et al. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997 Jul 17; 388(6639): 296–300
Liao F, Alkhatib G, Peden KW, et al. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med 1997 Jun 2; 185(11): 2015–23
Cocchi F, DeVico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995 Dec 15; 270(5243): 1811–5
Wang QC, Nie QH, Feng ZH. RNA interference: antiviral weapon and beyond. World J Gastroenterol 2003 Aug; 9(8): 1657–61
Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 2005 Jun; 23(6): 709–17
Lee SK, Dykxhoorn DM, Kumar P, et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood 2005 Aug 1; 106(3): 818–26
Chang LJ, Liu X, He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther 2005 Jul; 12(14): 1133–44
Hu WY, Myers CP, Kilzer JM, et al. Inhibition of retroviral pathogenesis by RNA interference. Curr Biol 2002 Aug 6; 12(15): 1301–11
Barnor JS, Miyano-Kurosaki N, Yamaguchi K, et al. Lentiviral-mediated delivery of combined HIV-1 decoy TAR and Vif siRNA as a single RNA molecule that cleaves to inhibit HIV-1 in transduced cells. Nucleosides Nucleotides Nucleic Acids 2005; 24(5–7): 431–4
Coburn GA, Cullen BR. Potent and specific inhibition of human immunodeficiency virus type 1 replication by RNA interference. J Virol 2002 Sep; 76(18): 9225–31
Lee MT, Coburn GA, McClure MO, et al. Inhibition of human immunodeficiency virus type 1 replication in primary macrophages by using Tat- or CCR5-specific small interfering RNAs expressed from a lentivirus vector. J Virol 2003 Nov; 77(22): 11964–72
Dave RS, Pomerantz RJ. Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains. J Virol 2004 Dec; 78(24): 13687–96
Li MJ, Kim J, Li S, et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol Ther 2005 Nov; 12(5): 900–9
Park J, Nadeau P, Zucali JR, et al. Inhibition of simian immunodeficiency virus by foamy virus vectors expressing siRNAs. Virology 2005 Dec 20; 343(2): 275–82
Park WS, Hayafune M, Miyano-Kurosaki N, et al. Specific HIV-1 env gene silencing by small interfering RNAs in human peripheral blood mononuclear cells. Gene Ther 2003 Nov; 10(24): 2046–50
Puerta-Fernandez E, Barroso-del Jesus A, Romero-Lopez C, et al. Inhibition of HIV-1 replication by RNA targeted against the LTR region. AIDS 2005 Jun 10; 19(9): 863–70
Novina CD, Murray MF, Dykxhoorn DM, et al. siRNA-directed inhibition of HIV-1 infection. Nat Med 2002 Jul; 8(7): 681–6
Park WS, Miyano-Kurosaki N, Hayafune M, et al. Prevention of HIV-1 infection in human peripheral blood mononuclear cells by specific RNA interference. Nucleic Acids Res 2002 Nov 15; 30(22): 4830–5
Kitabwalla M, Ruprecht RM. RNA interference: a new weapon against HIV and beyond. N Engl J Med 2002 Oct 24; 347(17): 1364–7
Martinez MA, Gutierrez A, Armand-Ugon M, et al. Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS 2002 Dec 6; 16(18): 2385–90
Qin XF, An DS, Chen IS, et al. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A 2003 Jan 7; 100(1): 183–8
Moore JP. Coreceptors: implications for HIV pathogenesis and therapy. Science 1997 Apr 4; 276(5309): 51–2
Nibbs RJ, Kriehuber E, Ponath PD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol 2001 Mar; 158(3): 867–77
Neil SJ, Aasa-Chapman MM, Clapham PR, et al. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol 2005 Aug; 79(15): 9618–24
Jacque JM, Triques K, Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature 2002 Jul 25; 418(6896): 435–8
Capodici J, Kariko K, Weissman D. Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J Immunol 2002 Nov 1; 169(9): 5196–201
Nishitsuji H, Ikeda T, Miyoshi H, et al. Expression of small hairpin RNA by lentivirus-based vector confers efficient and stable gene-suppression of HIV-1 on human cells including primary non-dividing cells. Microbes Infect 2004 Jan; 6(1): 76–85
Surabhi RM, Gaynor RB. RNA interference directed against viral and cellular targets inhibits human immunodeficiency virus type 1 replication. J Virol 2002 Dec; 76(24): 12963–73
Westerhout EM, ter Brake O, Berkhout B. The virion-associated incoming HIV-1 RNA genome is not targeted by RNA interference. Retrovirology 2006; 3: 57
Li CJ. Therapeutic biology: checkpoint pathway activation therapy, HIV Tat, and transkingdom RNA interference. J Cell Physiol 2006 Dec; 209(3): 695–700
Komano J, Miyauchi K, Matsuda Z, et al. Inhibiting the Arp2/3 complex limits infection of both intracellular mature vaccinia virus and primate lentiviruses. Mol Biol Cell 2004 Dec; 15(12): 5197–207
Kameoka M, Nukuzuma S, Itaya A, et al. RNA interference directed against Poly(ADP-Ribose) polymerase 1 efficiently suppresses human immunodeficiency virus type 1 replication in human cells. J Virol 2004 Aug; 78(16): 8931–4
Chiu YL, Cao H, Jacque JM, et al. Inhibition of human immunodeficiency virus type 1 replication by RNA interference directed against human transcription elongation factor P-TEFb (CDK9/CyclinT1). J Virol 2004 Mar; 78(5): 2517–29
Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol Cell Biol 2000 Apr; 20(8): 2629–34
Garrus JE, von Schwedler UK, Pornillos OW, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 2001 Oct 5; 107(1): 55–65
Stevenson M. Dissecting HIV-1 through RNA interference. Nat Rev Immunol 2003 Nov; 3(11): 851–8
Yu Z, Sanchez-Velar N, Catrina IE, et al. The cellular HIV-1 Rev cofactor hRIP is required for viral replication. Proc Natl Acad Sci U S A 2005 Mar 15; 102(11): 4027–32
Modem S, Badri KR, Holland TC, et al. Sam68 is absolutely required for Rev function and HIV-1 production. Nucleic Acids Res 2005; 33(3): 873–9
Liu S, Asparuhova M, Brondani V, et al. Inhibition of HIV-1 multiplication by antisense U7 snRNAs and siRNAs targeting cyclophilin A. Nucleic Acids Res 2004; 32(12): 3752–9
Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 2003 May; 4(5): 346–58
Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002 Apr 19; 296(5567): 550–3
Paddison PJ, Caudy AA, Bernstein E, et al. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 2002 Apr 15; 16(8): 948–58
Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 2005 Aug; 23(8): 1002–7
Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004 Nov 11; 432(7014): 173–8
Zimmermann TS, Lee AC, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature 2006 May 4; 441(7089): 111–4
Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet 2007 Mar; 8(3): 173–84
McNamara II JO, Andrechek ER, Wang Y, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol 2006 Aug; 24(8): 1005–15
Hu-Lieskovan S, Heidel JD, Bartlett DW, et al. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res 2005 Oct 1; 65(19): 8984–92
Zhou J, Swiderski P, Li H, et al. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res 2009; 37(9): 3094–109
Anderson J, Banerjea A, Akkina R. Bispecific short hairpin siRNA constructs targeted to CD4, CXCR4, and CCR5 confer HIV-1 resistance. Oligonucleotides 2003; 13(5): 303–12
Anderson J, Akkina R. HIV-1 resistance conferred by siRNA cosuppression of CXCR4 and CCR5 coreceptors by a bispecific lentiviral vector. AIDS Res Ther 2005 Jan 13; 2(1): 1
Anderson J, Akkina R. CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection. Retrovirology 2005; 2: 53
Kumar P, Ban HS, Kim SS, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 2008 Aug 22; 134(4): 577–86
Gitlin L, Karelsky S, Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 2002 Jul 25; 418(6896): 430–4
Michienzi A, Castanotto D, Lee N, et al. RNA-mediated inhibition of HIV in a gene therapy setting. Ann N Y Acad Sci 2003 Dec; 1002: 63–71
Singh SK. RNA interference and its therapeutic potential against HIV infection. Expert Opin Biol Ther 2008 Apr; 8(4): 449–61
Bennasser Y, Le SY, Benkirane M, et al. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity 2005 May; 22(5): 607–19
Williams BR. PKR: a sentinel kinase for cellular stress. Oncogene 1999 Nov 1; 18(45): 6112–20
Nekhai S, Jerebtsova M. Therapies for HIV with RNAi. Curr Opin Mol Ther 2006 Feb; 8(1): 52–61
Haasnoot J, de Vries W, Geutjes EJ, et al. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog 2007 Jun; 3(6): e86
Li WX, Li H, Lu R, et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc Natl Acad Sci U S A 2004 Feb 3; 101(5): 1350–5
Xu N, Segerman B, Zhou X, et al. Adenovirus virus-associated RNAII-derived small RNAs are efficiently incorporated into the RNA-induced silencing complex and associate with polyribosomes. J Virol 2007 Oct; 81(19): 10540–9
Boden D, Pusch O, Lee F, et al. Human immunodeficiency virus type 1 escape from RNA interference. J Virol 2003 Nov; 77(21): 11531–5
Das AT, Brummelkamp TR, Westerhout EM, et al. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J Virol 2004 Mar; 78(5): 2601–5
Jackson AL, Burchard J, Schelter J, et al. Widespread siRNA ‘off-target’ transcript silencing mediated by seed region sequence complementarity. RNA 2006 Jul; 12(7): 1179–87
Birmingham A, Anderson EM, Reynolds A, et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods 2006 Mar; 3(3): 199–204
Manche L, Green SR, Schmedt C, et al. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol Cell Biol 1992 Nov; 12(11): 5238–48
Singh SK, Girschick HJ. Toll-like receptors in Borrelia burgdorferi-induced inflammation. Clin Microbiol Infect 2006 Aug; 12(8): 705–17
Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 2005 Mar; 11(3): 263–70
Kim DH, Longo M, Han Y, et al. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat Biotechnol 2004 Mar; 22(3): 321–5
Marques JT, Devosse T, Wang D, et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol 2006 May; 24(5): 559–65
Robbins MA, Li M, Leung I, et al. Stable expression of shRNAs in human CD34+ progenitor cells can avoid induction of interferon responses to siRNAs in vitro. Nat Biotechnol 2006 May; 24(5): 566–71
Grimm D, Streetz KL, Jopling CL, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006 May 25; 441(7092): 537–41
An DS, Qin FX, Auyeung VC, et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther 2006 Oct; 14(4): 494–504
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S.K., Gaur, R.K. Progress towards Therapeutic Application of RNA Interference for HIV Infection. BioDrugs 23, 269–276 (2009). https://doi.org/10.2165/11317120-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11317120-000000000-00000