Abstract
Diabetes mellitus is a major risk factor for cardiovascular morbidity and mortality. This condition increases the risk of developing coronary, cerebrovascular and peripheral arterial disease up to 4-fold. Disease severity, as measured by chronic glycaemia, is associated with an increasing frequency of clinical events in each vascular bed. Several trials established that hyperglycaemia is the initiating cause of the diabetic tissue injury that we see in daily clinical practice. This process is modulated by genetic determinants of individual susceptibility and by independent accelerating factors such as hypertension and dyslipidaemia, but glycaemic control remains crucial for prevention of cardiovascular disease. The endothelium plays a key role in control of vascular tone by releasing endothelium-derived autacoids, the most important of which is nitric oxide (NO). Reduced NO bioavailability may represent an important triggering event in the initiation and progression of diabetic disease. The L-arginine/NO pathway is impaired at a number of sites in individuals with diabetes. Endothelial dysfunction is associated with a decrease in either basal or stimulated release of NO or there may be an increased breakdown of NO. In a high glucose setting, vascular cells present an increased generation of reactive oxygen species (ROS), with a consequent reduced bioavailability of NO.
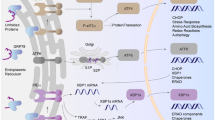
In this review we analyse the molecular mechanisms involved in the development and progression of diabetic vascular disease; to this purpose, we examine all the pathways, looking for common up- or down-stream events, the ‘unifying mechanism’, that gives us a more complete picture of diabetic disease. Moreover, we focus on the role of a small adaptor protein, p66shc, involved in the generation and regulation of ROS by mitochondria, referring to our experience in the field. Finally, we look at future perspectives and the promising field of stem cells.



Similar content being viewed by others
References
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997; 14Suppl. 5: S1–85
Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001; 286: 1195–200
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002; 287: 2570–81
Cosentino F, Luescher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol 1998; 32: 54–61
Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Radic Biol Mol 1994; 16: 383–91
Creager MA, Luscher TF, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108: 1527–32
Luscher TF, Creager MA, Beckman JA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation 2003; 108: 1655–61
Brunner H, Cockcroft JR, Deanfield J, et al. Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on endothelins and endothelial factors of the European Society of Hypertension. J Hypertens 2005; 23: 233–46
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. N Engl J Med 1993; 329: 977–86
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53
Shivalkar B, Dhondt D, Goovaerts I, et al. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol 2006; 97: 77–82
Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium-dependent vasodilatation in patients with insulin-dependent diabetes mellitus. Circulation 1993; 88: 2510–6
William SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilatation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1996; 27: 567–74
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329: 2002–12
McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium dependent and independent vasodilatation in patients with type 2 diabetes mellitus. Diabetologia 1992; 35: 771–6
Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004; 24: 816–23
Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res 2000; 87: 1123–32
Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 1999; 13: 23–30
Giardino I, Edelstein D, Brownlee M. Non enzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity: a model for intracellular glycosylation in diabetes. J Clin Invest 1994; 94: 110–7
Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859–66
Wells L, Hart GW. O-GlcNAc turns twenty: functional implication for posttranslational modification of nuclear and cytosolic protein with a sugar. FEBS Lett 2003; 546: 154–8
Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997; 416: 15–8
Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycaemic damage in endothelial cells. J Clin Invest 2003; 112: 1049–57
Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome C and p66shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005; 122: 221–33
Cosentino F, Hishikawa K, Katusic ZS, et al. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997; 96: 25–8
Cosentino F, Eto M, De Paolis P, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human aortic endothelial cells. Circulation 2003; 107: 1017–23
Kouroedov A, Eto M, Joch H, et al. Selective inhibition of protein kinase Cβ2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 2004; 110: 91–6
Haller H, Baur E, Quass P, et al. High glucose concentration and protein kinase C isoforms in vascular smooth muscle cells. Kidney Int 1995; 47: 1057–67
Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000; 404: 787–90
Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999; 402: 309–13
Francia P, delli Gatti C, Bachschmid M, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 2004; 110: 2889–95
Anversa P, Kajstura J, Leri A, et al. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation 2006; 113: 1451–63
Rota M, LeCapitaine N, Hosoda T, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66 shc gene. Circ Res 2006; 99: 42–52
Pacher P, Obrosova IG, Mabley JG, et al. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 2005; 12: 267–75
Johansen JS, Harris AK, Rychly DJ, et al. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005; 4: 5
Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycaemia attenuates endothelium-dependent vasodilatation in humans in vivo. Circulation 1998; 97: 1695–701
Beckman JA, Goldfine AB, Gordon MB, et al. Ascorbate restores endothelium-dependent vasodilatation impaired by acute hyperglycaemia in humans. Circulation 2001; 103: 1618–23
Haidara MA, Yassin HZ, Rateb M, et al. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol 2006; 4: 215–27
Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Vasc Biotechnol 2006; 7: 101–8
Meier JJ, Bhushan A, Butler PC. The potential for stem cell therapy in diabetes. Pediatr Res 2006; 56: R65–73
Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003; 348: 593–600
Wollert KC, Drexler H. Clinical application of stem cells for the heart. Circ Res 2005; 96: 151–63
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schiavoni, M., Cosentino, F., Camici, G.G. et al. Diabetes and Endothelial Dysfunction. High Blood Press Cardiovasc Prev 14, 5–10 (2007). https://doi.org/10.2165/00151642-200714010-00002
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2165/00151642-200714010-00002