Summary
Abstract
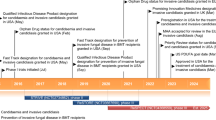
Intravenous micafungin (Mycamine®; Funguard®) is an echinocandin indicated in Japan and the EU for the treatment of pediatric patients (including neonates) with invasive candidiasis and as prophylaxis against Candida infection in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). In the EU, micafungin is also indicated in pediatric patients who are expected to have neutropenia for ≥10 days. In Japan, children may also receive micafungin for the treatment of, or as prophylaxis against, invasive Aspergillus infection. Micafungin is not currently approved for use in pediatric patients in the US.
Micafungin has very good antifungal activity against a wide range of Candida spp. in vitro. It has a favorable pharmacokinetic profile allowing for once-daily administration, has few drug-drug interactions, and reports of resistance are rare. The results of pediatric substudies indicate that intravenous micafungin is effective in a majority of patients for the treatment of candidemia and other types of invasive candidiasis, and provides effective prophylaxis against invasive fungal infections in pediatric patients undergoing HSCT. The tolerability profile of micafungin in pediatric patients was generally acceptable. In the EU, micafungin is indicated for use when other antifungal medications are not appropriate. Therefore, micafungin provides an alternative to other antifungal agents used in the management of candidemia and invasive candidiasis in pediatric patients, or as prophylaxis against fungal infections in pediatric patients undergoing HSCT.
Pharmacologic Properties
Micafungin inhibits the synthesis of 1,3-β-D-glucan, a major component of fungal cell walls. It has demonstrated in vitro antifungal and fungicidal activity against a wide range of Candida spp. encountered clinically, including multidrug-resistant Candida spp. residing in biofilms, and fluconazole-resistant Candida spp. In two large (n >1000 clinical isolates) studies, the minimum inhibitory concentration at which 90% of isolates were inhibited (MIC90) was 0.015–0.06 μg/mL for C. albicans, C. glabrata, C. kefyr, and C. tropicalis isolates. In the larger of the two studies, the micafungin MIC90 was 0.015–2 μg/mL across all Candida spp. isolates, and 100% susceptibility to micafungin was seen at an MIC of 0.06–2 μg/mL, depending on the isolate. An MIC susceptibility breakpoint for micafungin against Candida spp. of ≤2μg/mL was recently established by the Clinical and Laboratory Standards Institute. Reports of resistance to micafungin are rare.
Because of its high molecular weight, micafungin has poor oral bioavailability and is only available for intravenous administration. Over a dose range of 0.5–6.0 mg/kg, micafungin demonstrated linear, dose-proportional pharmacokinetics in pediatric patients with febrile neutropenia or deep mycosis and in premature neonates with a variety of underlying conditions. Micafungin is >99% plasma protein bound. It is mainly metabolized in the liver and is predominantly excreted via the fecal route. The clearance of micafungin was affected by age, with patients aged 2–8 years clearing the drug more quickly than those aged 9–17 years. Micafungin had few drug-drug interactions.
Therapeutic Efficacy
Intravenous micafungin was effective in the treatment of pediatric patients aged <16 years (including neonates) with candidemia or other types of invasive candidiasis in a pediatric substudy (n= 109) of a large (n = 537), randomized, double-blind, multicenter, phase III trial. In this substudy, the efficacy of micafungin 2 mg/kg/day in patients weighing ≤40 kg and 100 mg/day in those weighing >40 kg was compared with that of liposomal amphotericin B 3 mg/kg/day; all medications were infused over 1 hour each day for 2–8 weeks. Candidemia accounted for over 90% of infections in this study, and a non-C. albicans spp. was the infecting organism in ≈60% of cases. Micafungin was effective in the majority of patients for the treatment of candidemia or other types of invasive candidiasis, irrespective of patient age, primary diagnosis, baseline neutropenic status, and whether or not the patient required an increase in drug dosage, was born prematurely, or had an intravenous catheter present at baseline. With regard to the primary endpoint, 73% and 76% of micafungin and liposomal amphotericin B recipients achieved treatment success (i.e. clinical and mycologic response) at the end of treatment (EOT) as determined by the study investigator. Mycologic persistence at EOT occurred in 16% of patients in each treatment arm. Three patients in the micafungin group and no patients in the liposomal amphotericin B group had confirmed recurrence of fungal infection during the 12-week, post-treatment follow-up period.
Micafungin was also effective in the majority of patients as prophylaxis against invasive fungal infections in a subgroup of neutropenic pediatric patients (n = 84) undergoing HSCT who were included in a large (n = 882), randomized, double-blind, multicenter, phase III trial. Patients in this trial received micafungin 1 mg/kg/day (or 50 mg/day depending on patient weight) or intravenous fluconazole 8 mg/kg/day (or 400 mg/day depending on patient weight) over 1 hour for a mean duration of 23 days. In pediatric patients, treatment success (i.e. absence of systemic fungal infection; primary endpoint) occurred in 69% of micafungin recipients and 53% of fluconazole recipients. During the trial, one micafungin recipient and three fluconazole recipients developed proven or probable breakthrough infections.
Tolerability
The tolerability profile of micafungin was generally acceptable in pediatric patients with complex and life-threatening illnesses who received the drug for the treatment of invasive candidiasis or aspergillosis, or as prophylaxis against these infections. In addition, micafungin up to a dosage of 10 mg/kg/day appeared to be generally well tolerated in healthy neonates (n= 13). Data from a pediatric substudy (n= 106) also suggest that micafungin has a generally similar tolerability profile to that of liposomal amphotericin B in pediatric patients with candidemia or other types of invasive candidiasis.
The incidences of the most common adverse events associated with micafungin (i.e. infusion reactions, fever, and hypokalemia) were not significantly different from those reported with liposomal amphotericin B in the pediatric substudy. In a pooled analysis of data from pediatric patients in several clinical trials (n = 296), the most frequently occurring treatment-related adverse events were hypokalemia, increases in AST, ALT, bilirubin, or alkaline phosphatase levels, abnormal liver function tests, and hypertension. Elevations in creatinine, AST, ALT, and/or bilirubin from normal at baseline to above the upper limit of normal (ULN) or >2 or >2.5 × the ULN occurred in <25% of micafungin recipients and <20% of liposomal amphotericin B recipients in the pediatric substudy and ≥10% of micafungin recipients in the pooled analysis. Serious treatment-related adverse events occurred in 4% and 9% of patients in the pediatric substudy who received micafungin or liposomal amphotericin B. The rate of treatment discontinuations due to a treatment-related adverse event was 2% and 6% in micafungin and liposomal amphotericin B recipients. Seven treatment discontinuations occurred as a result of treatment-related adverse events associated with micafungin in the pooled analysis, and two of these were as a result of serious treatment-related adverse events.
Micafungin has been associated with isolated cases of more severe hepatic dysfunction, hepatitis, and hepatic failure. Pediatric patients appear more likely than adults to develop micafungin-associated liver function test abnormalities, and those aged <1 year are more likely to be affected than older pediatric patients. This may reflect the higher proportion of serious underlying disorders in the younger pediatric patients compared with the older children or adults.






Similar content being viewed by others
References
Groll AH, McNeil Grist L. Current challenge in the diagnosis and management of invasive fungal infections: report from the 15th International Symposium on Infections in the Immunocompromised Host: Thessaloniki; 2008 Jun 22–25. Int J Antimicrob Agents 2009 Feb; 33(2): 101–4
Cappelletty D, Eiselstein-McKitrick K. Reviews of therapeutics: the echinocandins. Pharmacology 2007; 27(3): 369–88
Fridkin SK, Kaufman D, Edwards JR, et al. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics 2006 May; 117(5): 1680–7
Almirante B, Rodríguez D. Antifungal agents in neonates: issues and recommendations. Pediatr Drugs 2007; 9(5): 311–21
Tortorano AM, Peman J, Bernhardt H, et al. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 2004 Apr; 23(4): 317–22
Stamos JK, Rowley AH. Candidemia in a pediatric population. Clin Infect Dis 1995 Mar; 20(3): 571–5
Levy I, Rubin LG, Vasishtha S, et al. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis 1998 May; 26(5): 1086–8
Zaoutis TE, Argon J, Chu J, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 2005 Nov; 41(9): 1232–9
Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 2003 Sep; 37(5): 634–43
Nucci M, Colombo AL, Silveira F, et al. Risk factors for death in patients with candidemia. Infect Control Hosp Epidemiol 1998 Nov; 19(11): 846–50
Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002 Aug; 110(2): 285–91
Faix RG. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. Pediatr Infect Dis J 1992 Feb; 11(2): 88–93
Saxen H, Virtanen M, Carlson P, et al. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr Infect Dis J 1995 Sep; 14(9): 776–81
Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. Pediatr Infect Dis J 2000 Apr; 19(4): 319–24
Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases (original studies). Pediatr Infect Dis J 1998 Jun; 17(6): 504–8
Faix RG, Kovarik SM, Shaw TR, et al. Mucocutaneous and invasive candidiasis among very low birth weight (<1,500 grams) infants in intensive care nurseries: a prospective study. Pediatrics 1989 Jan; 83(1): 101–7
Frattarelli D, Reed MD, Giacoia GP, et al. Antifungals in systemic neonatal candidiasis. Drugs 2004; 64(9): 949–68
Tawara S, Ikeda F, Maki K, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother 2000 Jan; 44(1): 57–62
Melkusová S, Bujdáková H, Volleková A, et al. The efficiency of the benzothiazole APB, the echinocandin micafungin, and amphotericin B in fluconazole-resistant Candida albicans and Candida dubliniensis [letter]. Pharmazie 2004; 59(7): 573–4
Ikeda F, Tanaka S, Ohki H, et al. Role of micafungin in the antifungal armamentarium. Curr Med Chem 2007; 14(11): 1263–75
Chandrasekar PH, Sobel JD. Micafungin: a new echinocandin. Clin Infect Dis 2006 Apr; 42(8): 1171–8
Groll AH, Walsh TJ. FK-463 Fujisawa Pharmaceutical Co Ltd. Curr Opin Anti Infect Drugs 2000; 2(4): 405–12
Astellas Pharma Inc. Funguard® 25 mg for infusion; Funguard® 50 mg for infusion; Funguard® 75 mg for infusion (micafungin sodium for injection): Japanese prescribing information [online]. Available from URL: http://www.e-search.ne.jp/~jpr/PDF/ASTELLAS13.PDF [Accessed 2008 Apr 8]
Astellas Pharma US Inc. Mycamine® (micafungin sodium) for injection: US prescribing information [online]. Available from URL: http://www.fda.gov/medwatch/safety/2007/Aug_PI/Mycamine_PI.pdf [Accessed 2008 Apr 8]
Astellas Pharma GmbH. Mycamine® 50 mg powder for solution for infusion: European prescribing information [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/mycamine/H-734-PI-en.pdf [Accessed 2008 May 16]
Cross SA, Scott LJ. Micafungin: a review of its use in adults for the treatment of invasive or oesophageal candidiasis, and as prophylaxis against Candida infection. Drugs 2008; 68(15): 2225–55
Ostrosky-Zeichner L, Pfaller M, Diekma D, et al. A proposal for an antifungal susceptibility testing (AST) breakpoint for micafungin (MFG) and Candida: integration of AST surveys and correlation with clinical outcomes [abstract no. M-2020]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Dannaoui E, Lortholary O, Raoux D, et al. In vitro activity of caspofungin and micafungin against 1038 yeasts isolates from France by EUCAST reference method [abstract no. P1974]. 17th European Congress of Clinical Microbiology and Infectious Diseases and 25th International Congress of Chemotherapy; y2007 Mar 31–Apr 3; Munich
Mochizuki N, Aibiki M, Matsumoto Y, et al. Evaluation of micafungin (MCFG) activity in serum, including serum synergism [abstract no. M-1598]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Mochizuki N, Ishikawa J, Matsumoto Y, et al. Evaluation of micafungin antifungal activities in patient serum [abstract no. M-1818]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Pai MP, Samples M, Mercier RC. Activity of flucytosine (F), micafungin (M), and voriconazole (V) in an in vitro pharmacodynamic model (PDM) of Candida spp. (CS) simulated endocardial vegetations (SEVs) [abstract no. M-1847]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Pai MP, Samples M, Mercier RC. Candida albicans (CA) biofilm activity of flucytosine (F), liposomal amphotericin B (L-AmB), and micafungin (M) in an in vitro pharmacodynamic model (PDM) of simulated endocardial vegetations (SEVs) [abstract no. M-1859]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; y2007 Sep 17–20; Chicago (IL)
Ali A, Vasquez G, Vager D, et al. Pharmacodynamics of echinocandins against Candida spp, including strains with reduced susceptibility to micafungin and caspofungin while retaining anidulafungin activity [abstract no. M-538]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Wierman M, Obeid K, Baxa D, et al. Emergence of Candida glabrata isolates with reduced susceptibility to caspofungin and micafungin, but not anidulafungin [abstract no. M-512]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Maki K, Morishita Y, Iguchi Y, et al. In vitro antifungal activity of FK463, a novel water-soluble echinocandin-like lipopeptide [abstract no. F-141]. 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1998 Sep 24–27; San Diego (CA)
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. 3rd ed. (document M27-A3). Wayne (PA): Clinical and Laboratory Standards Institute, 2008 Apr; 28 (14)
Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol 2008 Jan; 46(1): 150–6
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd informational supplement (document M27-S3). Wayne (PA): Clinical and Laboratory Standards Institute, 2008 Apr; 28 (15)
Takakura S, Fujihara N, Saito T, et al. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J Antimicrob Chemother 2004 Feb; 53(2): 283–9
Müller F-MC, Kurzai O, Hacker J, et al. Effect of the growth medium on the in vitro antifungal activity of micafungin (FK-463) against clinical isolates of Candida dubliniensis. J Antimicrob Chemother 2001 Nov; 48(5): 713–5
Ernst EJ, Roling EE, Petzold CR, et al. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother 2002 Dec; 46(12): 3846–53
Cantón E, Pemán J, Sastre M, et al. Killing kinetics of caspofungin, micafungin, and amphotericin B against Candida guilliermondii. Antimicrob Agents Chemother 2006 Aug; 50(8): 2829–32
Heyn K, Tredup A, Salvenmoser S, et al. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob Agents Chemother 2005 Dec; 49(12): 5157–9
Rodríguez MM, Ruiz M, Pastor FJ, et al. In vitro interaction of micafungin and fluconazole against Candida [letter]. J Antimicrob Chemother 2007 Jul; 60(1): 188–90
Mariné M, Serena C, Pastor J, et al. In vitro activity of micafungin combined with itraconazole against Candida spp. Int J Antimicrob Agents 2007 Nov; 30(5): 463–5
Kim M-N, Shin JH, Sung H, et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis 2009 Mar; 48(6): e57–61
Rodríguez-Tudela JL, Barchiesi F, Bille J, et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin Microbiol Infect 2003 Aug; 9(8): 1–8
Paderu P, Garcia-Effron G, Balashov S, et al. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob Agents Chemother 2007 Jun; 51(6): 2253–6
Kuhn DM, George T, Chandra J, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002 Jun; 46(6): 1773–80
Seidler M, Salvenmoser S, Müller F-MC. In vitro effects of micafungin against Candida biofilms on polystyrene and central venous catheter sections. Int J Antimicrob Agents 2006 Dec; 28(6): 568–73
Manavathu EK, Ramesh MS, Baskaran I, et al. A comparative study of the post-antifungal effect (PAFE) of amphotericin B, triazoles and echinocandins on Aspergillus fumigatus and Candida albicans. J Antimicrob Chemother 2004 Feb; 53(2): 386–9
Mariné M, Serena C, Pastor FJ, et al. Combined antifungal therapy in a murine infection by Candida glabrata. J Antimicrob Chemother 2006 Dec; 58(6): 1295–8
Olson JA, Adler-Moore JP, Smith PJ, et al. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob Agents Chemother 2005 Dec; 49(12): 4895–902
Hernandez S, López-Ribot JL, Najvar LK, et al. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother 2004 Apr; 48(4): 1382–3
Douglas CM, Foor F, Marrinan JA, et al. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-D-glucan synthase. Proc Natl Acad Sci U S A 1994 Dec 20; 91(26): 12907–11
Douglas CM, Marrinan JA, Li W, et al. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-D-glucan synthase. J Bacteriol 1994 Sep; 176(18): 5686–96
Douglas CM, D’Ippolito JA, Shei GJ, et al. Identification of the FKS1 gene of Candida albicans as the essential target of the 1,3-β-D-glucan synthase inhibitors. Antimicrob Agents Chemother 1997 Nov; 41(11): 2471–9
Park S, Kelly R, Nielsen Kahn J, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptiblity of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother 2005 Aug; 49(8): 3264–73
Laverdière M, Lalonde RG, Baril J-G, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother 2006 Apr; 57(4): 705–8
Schuetzer-Muehlbauer M, Willinger B, Krapf G, et al. The Candida albicans Cdr2p ATP-binding cassette (ABC) transporter confers resistance to caspofungin. Mol Microbiol 2003; 48(1): 225–35
Niimi K, Maki K, Ikeda F, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptiblity. Antimicrob Agents Chemother 2006 Apr; 50(4): 1148–55
Osherov N, May GS, Albert ND, et al. Overexpression of Sbe2p, a Golgi protein, results in resistance to caspfungin in Saccharomyces cerevisiae. Antimicrob Agents Chemother 2002 Aug; 46(8): 2462–9
Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 2004 Sep; 48(9): 3407–11
Chamilos G, Lewis RE, Albert N, et al. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob Agents Chemother 2007 Jun; 51(6): 2257–9
Jacobsen MD, Whyte JA, Odds FC. Candida albicans and Candida dubliniensis respond differently to echinocandin antifungal agents in vitro. Antimicrob Agents Chemother 2007 May; 51(5): 1882–4
Stevens DA, White TC, Perlin DS, et al. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis 2005 Mar; 51(3): 173–8
Clemons KV, Espiritu M, Parmar R, et al. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob Agents Chemother 2006 Apr; 50(4): 1293–7
Baixench M-T, Aoun N, Desnos-Ollivier M, et al. Acquired resistance to echinocandins in Candida albicans: case report and review. J Antimicrob Chemother 2007 Jun; 59(6): 1076–83
Mio T, Adachi-Shimizu M, Tachibana Y, et al. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in β-1,3-glucan synthesis. J Bacteriol 1997 Jul; 179(13): 4096–105
Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother 2006 Jul; 50(7): 2522–4
Moudgal V, Little T, Boikov D, et al. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother 2005 Feb; 49(2): 767–9
Messer SA, Diekema DJ, Boyken L, et al. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol 2006 Feb; 44(2): 324–6
Borg-von Zepelin M, Zaschke K, Gross U, et al. Effect of micafungin (FK463) on Candida albicans adherence to epithelial cells. Chemotherapy 2002 Jul; 48(3): 148–53
Gil-Lamaignere C, Salvenmoser S, Hess R, et al. Micafungin enhances neutrophil fungicidal functions against Candida pseudohyphae. Antimicrob Agents Chemother 2004 Jul; 48(7): 2730–2
Freire A, Arrieta A, Stevenson P, et al. Pharmacokinetics of micafungin in paediatric patients with invasive candidiasis and candidaemia [abstract no. A-772 plus poster]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother 2005 Aug; 49(8): 3317–24
Tabata K, Katashima M, Kawamura A, et al. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol Pharm Bull 2006 Aug; 29(8): 1706–11
Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J 2006 Dec; 25(12): 1110–5
Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 2009 May; 28(5): 412–5
Benjamin DK, Smith PB, Arrieta A, et al. Safety and pharmacokinetics (PK) of repeat-dose micafungin (MICA) in neonates [abstract no. A-012 plus poster]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington (DC)
Joseph JM, Jain R, Danziger LH. Micafungin: a new echinocandin antifungal. Pharmacotherapy 2007; 27(1): 53–67
Arrieta A. Overview of paediatric experience with micafungin [abstract]. 17th European Congress of Clinical Microbiology and Infectious Diseases and 25th International Congress of Chemotherapy; 2007 March 31–Apr 3; Munich
Steinbach WJ, Benjamin DK. New antifungal agents under development in children and neonates. Curr Opin Infect Dis 2005 Dec; 18(6): 484–9
Hope WW, Mickiene D, Petraitis V, et al. The pharmacokinetic and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 2008 Jan; 197(1): 163–71
Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol 2005 Oct; 45(10): 1145–52
Hebert MF, Blough DK, Townsend RW, et al. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol 2005 Sep; 45(9): 1018–24
Undre N, Stevenson P, Wilbraham D, et al. Amphotericin B does not affect the pharmacokinetics of micafungin (FK463) [abstract no. P971 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases and 25th International Congress of Chemotherapy; 2007 Mar 31–Apr 3; Munich
Undre N, Stevenson P, Brooks A, et al. Itraconazole does not affect the pharmacokinetics of micafungin (FK463) [abstract no. P970 plus poster]. 17th European Congress of Clinical Microbiology and Infectious Diseases and 25th International Congress of Chemotherapy; 2007 Mar 31–Apr 3; Munich
Hebert MF, Townsend RW, Austin S, et al. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol 2005 Aug; 45(8): 954–60
Keirns J, Sawamoto T, Holum M, et al. Steady-state pharmacokinetics of micafungin and voriconazole after separate and concomitant dosing in healthy adults. Antimicrob Agents Chemother 2007 Feb; 51(2): 787–90
Undre NA, Stevenson P, Amakye DD. Rifampicin and ritonavir do not affect the pharmacokinetics of micafungin (FK463), an echninocandin antifungal [abstract no. P1037]. Clin Microbiol Infect 2004 May; 10Suppl. 3: 279
Narumi S, Hakamada K, Toyoki Y, et al. Influence of antifungal agents on trough level of tacrolimus [abstract no. 1247]. Am J Transplant 2005; 5Suppl. 11: 474
Shimoeda S, Ohta S, Kobayashi H, et al. Analysis of the blood level of micafungin involving patients with hematological diseases: new findings regarding combination therapy with tacrolimus. Biol Pharm Bull 2005 Mar; 28(3): 477–80
Okugawa S, Ota Y, Tatsuno K, et al. A case of invasive central nervous system aspergillosis treated with micafungin with monitoring concentrations in the cerebrospinal fluid. Scand J Infect Dis 2007; 39(4): 344–83
Groll AH, Mickiene D, Petraitis V, et al. Compartmental pharmacokinetics and tissue distribution of the antifungal echinocandin lipopeptide micafungin (FK463) in rabbits. Antimicrob Agents Chemother 2001 Dec; 45(12): 3322–7
Niwa T, Yokota Y, Tokunaga A, et al. Tissue distribution after intravenous dosing of micafungin, an antifungal drug, to rats. Biol Pharm Bull 2004 Jul; 27(7): 1154–6
Krishna G, Vickery D, Ma L, et al. Effect of posaconazole on pharmacokinetics (PK) of caspofungin and micafungin [abstract no. A-015]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington (DC)
Sakaeda T, Iwaki K, Kakumoto M, et al. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J Pharm Pharmacol 2005 Jun; 57(6): 759–64
Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis. Pediatr Infect Dis J 2008 Sep; 27(9): 820–6
Kuse E-R, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007 May 5; 369(9572): 1519–27
van Burik J-A, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004 Nov 15; 39: 1407–16
European Medicines Agency. Assessment report for mycamine (EMEA/H/C/000734) [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/mycamine/H-734-en6.pdf [Accessed 2008 Aug 14]
Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect 2006 Nov; 53(5): 337–49
Arrieta A, Seibel NL, Walsh TJ, et al. Micafungin for the treatment of pediatric invasive fungal infections [abstract no. P313 plus poster]. 29th International Symposium on Intensive Care and Emergency Medicine; 2009 Mar 24–27; Brussels
Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis 2005 Oct; 24(10): 654–61
Arrieta A, Maddison P, Groll AH. Micafungin in pediatric patients: assessment of safety in clinical trials [abstract no. M-1162 plus poster]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Pappas PG. Invasive candidiasis. Infect Dis Clin North Am 2006 Sep; 20(3): 485–506
Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004 Jan; 38(2): 161–89
Herbrecht R, Flückiger U, Gachot B, et al. Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients. Eur J Cancer Suppl 2007 Jul; 5(2): 49–59
Merck Sharp & Dohme Ltd. Cancidas 50 mg powder for concentrate for solution for infusion: EU prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=12843 [Accessed 2009 Jan 12]
Gilead Sciences Ltd. Ambisome®: UK prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=1236 [Accessed 2008 Apr 8]
Cephalon Limited. Abelcet®: UK prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=2133 [Accessed 2008 Apr 8]
Beacon Pharmaceuticals. Amphocil™ 100mg: UK prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=18414 [Accessed 2008 Apr 8]
E. R. Squibb & Sons Limited. Fungizone® intravenous: UK prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=559 [Accessed 2008 Apr 8]
Pfizer Limited. Diflucan™ capsules 50mg and 200mg, powder for oral suspension 50mg/ml and 200mg/ml, intravenous infusion 2mg/ml [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/dis-playDocPrinterFriendly.asp?documentid=1459 [Accessed 2008 Apr 8]
Hospira UK Ltd. Fluconazole 2mg/ml intravenous infusion [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=16792 [Accessed 2008 Apr 8]
Valeant Pharmaceuticals Ltd. Ancotil® 2.5g/250 ml solution for infusion: UK prescribing information [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=6215 [Accessed 2008 Apr 8]
Pfizer Limited. Vfend® 50mg and 200mg film-coated tablets, Vfend® 200mg powder for solution for infusion, Vfend® 40mg/ml powder for oral suspension [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=10059 [Accessed 2008 Apr 8]
Wong-Beringer A, Kriengkauykiat J. Systemic antifungal therapy: new options, new challenges. Pharmacotherapy 2003; 23(11): 1441–62
Blyth CC, Palasanthiran P, O’Brien TA. Antifungal therapy in children with invasive fungal infections: a systematic review. Pediatrics 2007 Apr; 119(4): 772–84
X-Gen Pharmaceuticals Inc. Amphotericin B for injection USP: US prescribing information [online]. Available from URL: http://www.x-gen.us/Injectables/Amphotericin%20B/Amphotericin_files/insert/Amphotericin_pi.pdf [Accessed 2008 Apr 8]
Slavin MA, Szer J, Grigg AP, et al. Guidelines for the use of antifungal agents in the treatment of invasive Candida and mould infections. Intern Med J 2004 Apr; 34(4): 192–200
Pfizer Inc. Vfend® I.V. (voriconazole) for injection, Vfend® tablets (voriconazole), Vfend® (voriconaole) for oral suspension [online]. Available from URL: http://www.fda.gov/cder/foi/label/2008/021266s023,021267s024,021630s013lbl.pdf [Accessed 2008 Apr 8]
Denning DW, Kibbler CC, Barnes RA. British Society for Medical Mycology proposed standards of care for patients with invasive fungal infections. Lancet Infect Dis 2003 Apr; 3(4): 230–40
Böhme A, Ruhnke M, Buchheidt D, et al. Treatment of fungal infections in hematology and oncology: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2003 Oct; 82Suppl. 2: S133–40
Maertens JA, Frère P, Lass-Flörl C, et al. Primary antifungal prophylaxis in leukaemia patients. Eur J Cancer 2007 Jul; 5(2): 43–8
Maertens J, Frère P, Lass-Flörl C, et al. 2007 update of the ECIL-1 guidelines for antifungal prophylaxis in leukemia patients, including allogeneic HSCT recipients: oral presentation [online]. Available from URL: http://www.eortc.be/services/unit/idg/documents/08.Antifungalprophylaxis.pdf [Accessed 2008 Sep 10]
Centers for Disease Control and Prevention, Infectious Diseases Society of America, and the American Society of Blood and Bone Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 2000 Dec; 6(6 Suppl. 1): 7–83
Pfizer Inc. Ecalta® 100 mg powder and solvent for concentrate for solution for infusion: EU prescribing information [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/ecalta/H-788-PI-en.pdf [Accessed 2008 Sep 16]
Merck & Co. Inc. Cancidas® (caspofungin acetate) for injection: highlights of US prescribing information [online]. Available from URL: http://www.fda.gov/cder/foi/label/2008/021227s021lbl.pdf [Accessed 2009 Jan 12]
Pfizer Inc. Eraxis™ (anidulafungin) for injection: US prescribing information [online]. Available from URL: http://www.fda.gov/cder/foi/label/2007/021632s006lbl.pdf [Accessed 2009 Jan 12]
Cornely OA, Sidhu M, Odeyemi I, et al. Economic analysis of micafungin versus liposomal amphotericin B for treatment of candidaemia and invasive candidiasis in Germany. Curr Med Res Opin 2008; 24(6): 1743–53
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: I.W. Fong, Division of Infectious Diseases, University of Toronto, Toronto, Ontario, Canada; A.H. Groll, Center for Bone Marrow Transplantation and Department of Pediatric Hematology and Oncology, University Children’s Hospital, Muenster, Germany; P. Palasanthiran, Department of Immunology and Infectious Diseases, Sydney Children’s Hospital, Randwick, New South Wales, Australia; T. Rogers, Department of Clinical Microbiology, Trinity College Dublin, Dublin, Ireland; A. Shetty, Department of Medical Microbiology, Cwm Taf NHS Trust, Wales, UK.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘micafungin’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health ∣ Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE, and AdisBase search terms were ‘micafungin’ and (‘children’ or ‘infant’ or ‘neonatal’ or ‘pediatric’ or ‘paediatric’). Searches were last updated 18 June 2009.
Selection: Studies in patients with candidemia or invasive candidiasis who received micafungin. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Micafungin, candidemia, invasive candidiasis, candida, adolescents, children, infants, neonates, pediatric, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Carter, N.J., Keating, G.M. Micafungin. Pediatr-Drugs 11, 271–291 (2009). https://doi.org/10.2165/00148581-200911040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00148581-200911040-00006