Abstract
Respiratory distress syndrome (RDS) in preterm neonates is caused by a lack of alveolar surfactant, which leads to decreased pulmonary compliance and increased work of breathing. Effective therapy for RDS has reduced mortality at the expense of increasing the number of preterm survivors with chronic lung disease. Drugs such as corticosteroids, proterelin (thyrotropin-releasing hormone) and ambroxol have all been administered to mothers to promote fetal lung maturation, but of these only corticosteroids have been proven to be of benefit.
The management of RDS includes assisted ventilation and surfactant replacement therapy. There are several surfactant preparations, some synthetic and others derived from animal lungs, and recent research has been directed at finding which, if any, is superior. The timing of the first dose has also been studied. Prophylactic surfactant administration within the first 15 minutes of life appears to be more efficacious than later treatment for very preterm babies, but could lead to some neonates being treated unnecessarily and perhaps being exposed to adverse effects.
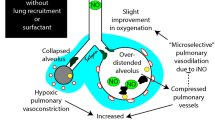
Newer treatments for neonates with RDS are aimed at reducing the pulmonary inflammation that occurs as a result of ventilatory barotrauma and oxygen toxicity. Superoxide dismutase, along with other antioxidants, may be beneficial as a free radical scavenger to reduce oxygen toxicity. Inhaled nitric oxide may reduce oxygen requirements by reducing ventilation-perfusion mismatching, and early treatment with corticosteroids may reduce pulmonary inflammation. All of these treatments are currently undergoing clinical trials.
Similar content being viewed by others
References
Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child 1959; 97: 517–23
Halliday HL. Pulmonary disorders and apriea. In: Campbell AGM, McIntosh N, editors. Forfar and Arneil’s textbook of pediatrics. 5th ed. Churchill Livingstone, 1998: 177–82
Abman SH, Groothuis JR. Pathophysiology and treatment of bronchopulmonary dysplasia. Pediatr Clin North Am 1994; 41: 277–315
Escobedo MB, Gonzales A. Bronchopulmonary dysplasia in the tiny infant. Clin Perinatol 1986; 13: 315–26
Liggins GC, Howie RN. A controlled trial of antepartum gluco-corticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972; 50: 515–25
Crowley P. Corticosteroids prior to preterm delivery [Cochrane review]. In: The Cochrane Library. Issue 2. Oxford: Update Software, 1998
Garite TJ, Rumny PJ, Briggs GG, et al. A randomized, placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24 to 28 weeks’ gestation. Am J Obstet Gynecol 1992; 166: 646–51
Silver RK, Vyskocil C, Solomon SL, et al. Randomized trial of antenatal dexamethasone in surfactant-treated infants delivered before 30 weeks’ gestation. Obstet Gynecol 1996; 87: 683–91
Clyman RI, Ballard PL, Sniderman S, et al. Prenatal administration of betamethasone for prevention of patent ductus arteriosus. J Pediatr 1981; 98: 123–6
Jobe AH, Mitchell BR, Gunkel JH. Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant on preterm infants. Am J Obstet Gynecol 1993; 168: 508–13
Van Marter LJ, Leviton A, Kuban KCK, et al. Maternal glucocorticoid therapy and reduced risk of bronchopulmonary dysplasia. Pediatrics 1990; 86: 331–6
Kass EN, Finland M. Corticosteroids and infection. Adv Intern Med 1958; 9: 45–52
Jacobs MM, Knight AB, Arias F. Maternal pulmonary edema resulting from betamimetic and glucocorticoid therapy. Obstet Gynecol 1980; 56: 56–9
Barker DJ. Fetal origins of coronary heart disease. BMJ 1995; 311: 171–4
Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998; 351: 173–7
De Souza SW, Adlard BPF. Growth of suckling rats after treatment with dexamethasone or cortisol. Arch Dis Child 1973; 48: 519–22
Ikegami M, Jobe AH, Newman J, et al. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Crit Care Med 1997; 156: 178–84
Johnson JWC, Mitzner W, Beck JC, et al. Long term effects of betamethasone on fetal development. Am J Obstet Gynecol 1981; 141: 1053–64
Gumbinas M, Oda M, Huttenlocher P. The effects of corticosteroids on myelination of the developing rat brain. Biol Neonate 1973; 22: 355–66
MacArthur BA, Howie RN, Dezoete JA, et al. Cognitive and psychosocial development of 4-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics 1981; 68: 638–43
MacArthur BA, Howie RN, Dezoete JA, et al. School progress and cognitive development of 6-year-old children whose mothers were treated with antenatal betamethasone. Pediatrics 1982; 70: 99–105
Hagan R, French N, Evans S, et al. Repeated antenatal corticosteroids: growth and early childhood outcome [abstract]. Pediatr Res 1997; 42: 405A
Ballard PL. Hormones and lung maturation. Monogr Endocrinol 1986; 28: 197–346
Ballard PL, Hovey ML, Gonzales LK. Thyroid hormone stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung. J Clin Invest 1984; 74: 898–905
Wu B, Kikkawa M, Orzalesi E, et al. The effect of thyroxine on the maturation of fetal rabbit lungs. Biol Neonate 1973; 22: 161–8
Ikegami M, Jobe A, Pettenazzo A, et al. Effects of maternal treatment with corticosteroids, T3, TRH, and their combinations on lung function of ventilated preterm rabbits with and without surfactant treatments. Am Rev Respir Dis 1987; 136: 892–8
Schellenberg JC, Liggins GC, Manzai M, et al. Synergistic hormonal effects on lung maturation in fetal sheep. J Appl Physiol 1988; 65: 94–100
Moya F, Mena P, Huesser P, et al. Response of the maternal, fetal and neonatal pituitary-thyroid axis to thyrotropin releasing hormone. Pediatr Res 1986; 20: 982–6
Morales W, O’Brien W, Angel J, et al. Fetal lung maturation: the combined use of corticosteroids and thyrotropin releasing hormone. Obstet Gynecol 1989; 73: 111–6
Ballard RA, Ballard PL, Creasy R, et al. Respiratory disease in very low birth weight infants after prenatal thyrotropin releasing hormone and glucocorticoid. Lancet 1992; 339: 510–5
Knight DB, Liggins GC, Wealthall SR. A randomised, controlled trial of antepartum thyrotropin releasing hormone and betamethasone in the prevention of respiratory distress in pre-term infants. Am J Obstet Gynecol 1994; 171: 11–6
ACTOBAT Study Group. Australian collaborative trial of antenatal thyrotropin releasing hormone (ACTOBAT) for prevention of neonatal respiratory disease. Lancet 1995; 345: 877–82
Ballard RA, Ballard PL, Cnaan A, et al. Antenatal thyrotropin-releasing hormone to prevent lung disease in preterm infants. N Engl J Med 1998; 338: 493–8
Crowther CA, Hiller JE, Haslam RR, et al. Australian Collaborative Trial of Antenatal Thyrotropin Releasing Hormone: adverse effects at 12 month follow up. Pediatrics 1997; 99: 311–7
Kimya Y, Kucukkomurcu S, Ozan H, et al. Antenatal ambroxol usage in the prevention of infant respiratory distress syndrome: beneficial and adverse effects. Clin Exp Obstet Gynecol 1995; 22: 204–11
Luerti M, Lazzarin A, Corbella E, et al. An alternative to steroids for prevention of respiratory distress syndrome (RDS): multicentre controlled study to compare ambroxol and betamethasone. J Perinat Med 1987; 15: 227–38
Dani C, Grella PV, Lazzarin L, et al. Antenatal ambroxol treatment does not prevent the respiratory distress syndrome in premature infants. Eur J Pediatr 1997; 156: 392–3
Halliday HL. Which interventions for neonatal respiratory failure are effective? Croat Med J 1998; 39: 165–70
Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline membrane disease. Lancet 1980; I: 55–9
Soll RF. Prophylactic administration of natural surfactant extract [Cochrane review]. In: The Cochrane Library. Issue 2. Oxford: Update Software, 1998
Howlett A, Ohlsson A. Inositol in preterm infants with RDS. [Cochrane review]. In: The Cochrane Library. Issue 1. Oxford: Updated Software, 1998
Northway WH. Bronchopulmonary dysplasia: then and now. Arch Dis Child 1990; 65: 1076–81
Avery ME, Tooley WH, Keller JB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987; 79: 26–30
Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airways pressure for newborns with respiratory distress syndrome. N Engl J Med 1994; 331: 1051–5
Moen A, Xu XQ, Rootwelt T, et al. Acute effects on systemic and pulmonary hemodynamics of intratracheal instillation of porcine surfactant or saline in surfactant depleted newborn piglets. Pediatr Res 1997; 41: 486–92
Halliday HL. Surfactant therapy in neonates. Pediatr Perinat Drug Ther 1997; 1: 30–40
Skinner J. The effects of surfactant on haemodynamics in hyaline membrane disease. Arch Dis Child 1997; 76: F67–9
OSIRIS Collaborative Group. Early versus delayed neonatal administration of a synthetic surfactant: the judgement of OSIRIS. Lancet 1992; 340: 1363–9
Couser RJ, Ferrara TB, Wright GB, et al. Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. J Pediatr 1996; 128: 631–7
Van Overmeire B, Follens I, Hartmann S, et al. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed 1997; 76: F179–84
Hellstrom-Westas L, Bell AH, Skov L, et al. Cerebroelectrical depression following surfactant treatment in preterm neonates. Pediatrics 1992; 89: 643–7
Cowan F, Whitelaw A, Wertheim D, et al. Cerebral blood flow velocity changes after rapid administration of surfactant. Arch Dis Child 1991; 66: 1105–9
Horbar JD, Soll RF, Schachinger H, et al. A European multi-centre randomised controlled trial of single dose surfactant therapy for idiopathic respiratory distress syndrome. Eur J Pediatr 1990; 149: 416–23
Halliday HL. Follow up data from babies treated with surfactant. In: Bevilaqua G, Parmigiani S, Robertson B, editors. Surfactant in clinical practice. Chur: Harwood Academic Publishers, 1992: 149–56
Long W. Synthetic surfactant. Semin Perinatol 1993; 17: 275–84
Disse B, Weller E, Lutzen L, et al. Comparison between natural and artificial surfactant preparations in premature rabbit fetuses. In: Lachmann B, editor. Surfactant replacement therapy. Berlin: Springer, 1989: 42–6
Schrod L, Hornemann F, von Stockhausen HB. Chemiluminescence activity of phagocytes from tracheal aspirates of premature infants after surfactant therapy. Acta Paediatr 1996; 85: 719–23
Herting E, Jarstrand C, Rasool O, et al. Experimental neonatal group B streptococcal pneumonia: effect of a modified porcine surfactant on bacterial proliferation in ventilated near-term rabbits. Pediatr Res 1994; 36: 784–91
Baur FM, Brenner B, Goetze-Speer B, et al. Natural porcine surfactant (Curosurf®) down-regulates mRNA of tumor necrosis factor-α (TNF-α) and TNF-α type II receptor in lipopolysaccharide-stimulated monocytes. Pediatr Res 1998; 44: 32–6
Leichty EA, Donovan E, Purohit D, et al. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics 1991; 88: 19–28
Chida S, Phelps DS, Soll RF, et al. Surfactant proteins and anti-surfactant antibodies in sera from infants with respiratory distress syndrome with and without surfactant treatment. Pediatrics 1991; 88: 84–9
Halliday HL. Natural vs synthetic surfactants in neonatal respiratory distress syndrome. Drugs 1996; 51: 226–37
Soll RF. Natural surfactant vs synthetic surfactant in the treatment of established respiratory distress syndrome [Cochrane review]. In: The Cochrane Library. Issue 2. Oxford: Update Software, 1998
Speer CP, Gefeller O, Groneck P, et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child 1995; 72: F8–13
Bloom BT, Kattwinkel J, Hall RT, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics 1997; 100: 31–8
Whitelaw A. Controversies: synthetic or natural surfactant treatment for respiratory distress syndrome? The case for synthetic surfactant. J Perinat Med 1996; 24: 427–35
Morley CJ. Systematic review of prophylactic vs rescue surfactant. Arch Dis Child 1997; 77: F70–4
Soll RF, Morley CJ. Prophylactic surfactant vs treatment with surfactant [Cochrane review]. In: The Cochrane Library. Issue 2. Oxford: Update Software, 1998
Kendig JW, Ryan RM, Sinkin RA, et al. Comparison of two strategies for surfactant prophylaxis in very premature infants: a multicenter randomised trial. Pediatrics 1998; 101: 1006–12
Corcoran JD, Sheehan O, O’Hare M, et al. Tracheal surfactant protein A following treatment with Curosurf. J Appl Cardiopulm Pathophysiol 1995; 5: 245–8
Speer CP, Robertson B, Curstedt T, et al. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics 1992; 89: 13–20
Dunn MS, Shennan AT, Possmayer F. Single versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks gestation with respiratory distress syndrome. Pediatrics 1990; 86: 564–71
Halliday HL, Tarnow-Mordi WO, Corcoran JD, et al. Multi-centre randomised trial comparing high and low dose surfactant regimens for the treatment of respiratory distress syndrome (the Curosurf 4 trial). Arch Dis Child 1993; 69: 276–80
Periera GR, Baker L, Egler J, et al. Serum myoinositol concentrations in premature infants fed human milk, formula for infants, and parenteral nutrition. Am J Clin Nutr 1990; 51: 589–93
Bromberger P, Hallman M. Myoinositol in small preterm infants: relationship between intake and serum concentration. J Pediatr Gastroenterol Nutr 1986; 5: 455–8
Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: relationship between serum concentration, renal excretion, and lung effluent phospholipids. J Pediatr 1987; 110: 604–10
Wauer RR, Schmalisch G, Bohme B, et al. Randomized double blind trial of ambroxol for the treatment of respiratory distress syndrome. Eur J Pediatr 1992; 151: 357–63
Schmalisch G, Wauer RR, Bohme B. Changes in pulmonary function in preterm infants recovering from RDS following early treatment with ambroxol: results of a randomised trial. Pediatr Pulmonol. In press
Finer NN, Barrington KJ. Nitric oxide in respiratory failure in the newborn infant. Semin Perinatol 1997; 21: 426–40
Hogman M, Frostell C, Arnberg H, et al. Bleeding time prolongation and NO inhalation. Lancet 1993; 341: 1664–5
Hallman M, Waffarn F, Bry K, et al. Surfactant dysfunction after inhalation of nitric oxide. J Appl Physiol 1996; 80: 2026–34
Robbins CG, Davis JM, Merritt TA, et al. Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol 1995; 269: L545–50
Subhedar NV, Ryan SW, Shaw NJ. Open randomised controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child 1997; 77: F185–90
Subhedar NV, Shaw NJ. Changes in oxygenation and pulmonary haemodynamics in preterm infants treated with inhaled nitric oxide. Arch Dis Child 1997; 77: F191–7
Meurs KP, Rhine WD, Asselin JM, et al. Response of premature infants with severe respiratory failure to inhaled nitric oxide. Pediatr Pulmonol 1997; 24: 319–23
Holm BA, Notter RH, Siegle J, et al. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol 1985; 59: 1402–9
Frank L, Sosenko IRS. Development of lung antioxidant enzyme system in late gestation: possible implications for the prematurely born infant. J Pediatr 1987; 110: 9–14
Frank L, Sosenko IRS. Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res 1991; 29: 292–6
Autor AP, Frank L, Roberts RJ. Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatr Res 1976; 10: 154–8
Rosenfeld W, Evans H, Concepcion L, et al. Prevention of bronchopulmonary dysplasia by administration of bovine superoxide dismutase in preterm infants with respiratory distress syndrome. J Pediatr 1984; 105: 781–5
Davis JM, Rosenfeld WN, Sanders RJ, et al. Prophylactic effects of recombinant human superoxide dismutase in neonatal lung injury. J Appl Physiol 1993; 74: 2234–41
Rosenfeld WN, Davis JM, Parton L, et al. Safety and pharmacokinetics of recombinant human superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 1996; 97: 811–7
Davis JM, Rosenfeld WN, Richter SE, et al. Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 1997; 100: 24–30
Baden M, Bauer C, Colle E, et al. Plasma corticosteroids in infants with the respiratory distress syndrome. Pediatrics 1973; 52: 782–7
Watterberg KL, Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics 1995; 95: 120–5
Baden M, Bauer C, Colle E, et al. A controlled trial of hydrocortisone therapy in infants with respiratory distress syndrome. Pediatrics 1972; 50: 526–34
Mammel MC, Green TP, Johnson DE, et al. Controlled trial of dexamethasone in infants with bronchopulmonary dysplasia. Lancet 1983; I: 1356–8
Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo-controlled trial. Pediatrics 1991; 88: 421–7
Ng PC. The effectiveness and side-effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Arch Dis Child 1993; 68: 330–6
Gramsbergen A, Mulder EJH. The influence of betamethasone and dexamethasone on motor development in young rats. Pediatr Res 1998; 44: 105–10
Halliday HL. Areview of postnatal corticosteroids for treatment and prevention of chronic lung disease in the preterm infant. Prenatal Neonat Med 1997; 2: 237–48
Halliday HL. Postnatal corticosteroids for prevention of chronic lung disease in the preterm infant: early treatment (<96 hours) [Cochrane review]. In: The Cochrane Library. Issue 3. Oxford: Update Software, 1998
Bhuta T, Ohlsson A. Systematic review and meta-analysis of early postnatal dexamethasone for prevention of chronic lung disease. Arch Dis Child Fetal Neonatal Ed 1998; 79: F26–33
NIH Consensus Development Panel. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995; 273: 413–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sweet, D.G., Halliday, H.L. Current Perspectives on the Drug Treatment of Neonatal Respiratory Distress Syndrome. Pediatr-Drugs 1, 19–30 (1999). https://doi.org/10.2165/00128072-199901010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128072-199901010-00003