Abstract
Objective: This study aimed to evaluate the penetration into gynaecological tissues of ulifloxacin, the active metabolite of prulifloxacin, a once-daily fluoroquinolone administered once or in repeated doses.
Methods: This was an open-label, randomised study that included 20 consenting female inpatients (age range 40–65 years) requiring total simple hysterectomy as a result of benign disease. Three groups of patients were enrolled: group A (four patients whose gynaecological tissue samples were used to set up the bioanalytical method); group B (eight patients treated 3 hours before surgery with one 600mg tablet of prulifloxacin); group C (eight patients treated with prulifloxacin 600mg once daily for 3 days and undergoing surgery 3 hours after the last dose). Patients to be treated with prulifloxacin were randomly allocated to group B or C. During surgery, samples of blood were collected jointly with healthy tissue removed from the endometrium, proximal fallopian tube, vaginal posterior fornix and portio vaginalis. Ulifloxacin concentrations in plasma and gynaecological tissues were determined by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) bioanalytical method. An intrastudy assessment of the bioanalytical method performance was also conducted for plasma and tissues using calibration and quality control data (spiked samples).
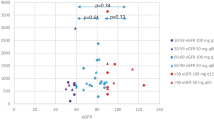
Results: Ulifloxacin mean concentrations were always higher in group C than in group B patients, both in plasma (0.76 vs 0.53 μg/mL) and in gynaecological tissues, namely fallopian tube (1.38 vs 0.81 μg/g), posterior fornix (1.48 vs 1.05 μg/g), portio vaginalis (1.46 vs 1.45 μg/g) and endometrium (2.20 vs 1.39 μg/g), as expected after repeated drug administrations. Tissue concentrations observed after repeated administrations were generally higher than the ulifloxacin minimum inhibitory concentrations for pathogens more frequently involved in gynaecological bacterial infections. The mean tissue/plasma ratios ranged between 1.5 and almost 3.
Conclusion: The results of this study are promising but not fully predictive of clinical or microbiological efficacy for prulifloxacin. There is a need for appropriate clinical trials confirming that prulifloxacin is a useful therapeutic tool in patients with gynaecological bacterial infections.







Similar content being viewed by others
References
Keam SJ, Perry CM. Prulifloxacin. Drugs 2004; 64 (19): 2221–34
Tougou K, Nakamura A, Watanabe S, et al. Paraxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab Dispos 1998; 26 (4): 355–9
Picollo R, Brion N, Gualano V, et al. Pharmacokinetics and tolerability of prulifloxacin after single oral administration. Arzneimittelforschung 2003; 53 (3): 201–5
Nakashima M, Uematsu T, Kosuge K, et al. Pharmacokinetics and safety of NM441, a new quinolone, in healthy male volunteers. J Clin Pharmacol 1994; 34: 930–7
Gutman RE, Peipert JF, Weitzen S, et al. Evaluation of clinical methods for diagnosing bacterial vaginosis. Obstet Gynecol 2005; 105 (3): 551–6
Donders GGG, Vereecken A, Bosmans E, et al. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 2002; 109: 34–43
Leitich H, Kiss H. Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2007 Jun; 21 (3): 375–90
Mikamo H, Kawazoe K, Izumi K, et al. NM441: penetration into gynaecological tissues and in vitro activity against clinical isolates from obstetric and gynaecological patients. Drugs 1995; 49 Suppl. 2: 326–30
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41
Concia E, Allegranzi B, Ciottoli GB, et al. Penetration of orally administered prulifloxacin into human lung tissue. Clin Pharmacokinet 2005; 44 (12): 1287–94
Mikamo H, Ninomiya M, Tamaya T. Penetration of oral telithromycin into female genital tissues. J Infect Chemother 2003; 9 (4): 358–60
Gerstner GJ, Dalhoff A, Weuta H. Single and multiple dose pharmacokinetics of ciprofloxacin in gynecological tissues. Infection 1988; 16 Suppl. 1: S24–8
Lehtonen L, Wikberg R, Karhunen M, et al. Erythromycin and 2′-acetyl erythromycin concentrations in plasma and pelvic tissues after repeated doses of erythromycin acistrate. Arzneimittelforschung 1993; 43 (1): 53–6
Martens MG, Maccato M, Van Hook C, et al. Penetration of trovafloxacin into gynecologic tissues. Am J Surg 1998; 176 (6A Suppl.): 18S–22S.
Montanari MP, Mingoia M, Varaldo PE. In vitro antibacterial activities of AF 3013, the active metabolite of prulifloxacin, against nosocomial and community Italian isolates. Antimicrob Agents Chemother 2001; 45 (12): 3616–22
Prats G, Roig C, Mirò E, et al. In vitro activity of the active metabolite of prulifloxacin (AF 3013) compared with six other fluoroquinolones. Eur J Clin Microbiol Infect Dis 2002; 21 (4): 328–34
Wispelwey B. Clinical implications of pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Infect Dis 2005; 41 Suppl. 2: S127–35
Barger A, Fuhst C, Wiedemann B. Pharmacological indices in antibiotic therapy. J Antimicrob Chemother 2003; 52 (6): 893–8
Acknowledgements
This study was supported by a grant from Angelini Pharmaceuticals ACRAF SpA, Rome, Italy. Drs Rosignoli, Picollo, Ruggieri and Dionisio are employees of Angelini Pharmaceuticals ACRAF SpA; the other authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorlero, F., Lorenzi, P., Rosignoli, M.T. et al. Penetration of Prulifloxacin into Gynaecological Tissues after Single and Repeated Oral Administrations. Drugs R D 8, 373–381 (2007). https://doi.org/10.2165/00126839-200708060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200708060-00005