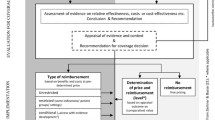
Abstract
Because of growing pressure on the healthcare budget in The Netherlands, appropriate justification of current expenditures and future investments in public healthcare are becoming increasingly important. Therefore, the Dutch Ministry of Health, Welfare and Sport is expanding its use of pharmacoeconomic evaluation in informed reimbursement decision-making of new pharmaceuticals. Since June 2002, pharmaceutical companies have been invited to submit a pharmacoeconomic dossier with their reimbursement applications of innovative drugs. As of January 2005 submission of a pharmacoeconomic dossier is mandatory for all drugs claiming to have therapeutic value.
Currently, several European governmental and non-governmental organisations are making efforts to harmonise pharmacoeconomic research guidelines at the EU level. Ultimately, this may facilitate a more efficient way of conducting pharmacoeconomic research and encourage the use of pharmacoeconomic data by national assessment agencies and governments.
It is anticipated that international pharmaceutical companies will increasingly invest in pharmacoeconomics while government staff will become more experienced in appraising the dossiers, thus resulting in an upward momentum in the quality and usability of pharmacoeconomic data.
From the Dutch government’s perspective, the use of pharmacoeconomic evaluation in reimbursement decision-making should offer a true opportunity for pharmaceutical companies to present the added value for money of new drugs. Using pharmacoeconomic data, costs, benefits and effects of pharmaceuticals are increasingly being considered from a societal perspective, thus going beyond the sole consideration of the impact on the pharmaceutical budget.

Similar content being viewed by others
References
Ministerie van Volksgezondheid Welzijn en Sport. Medicines policy in The Netherlands: International Publication Series health, Welfare and Sport no. 15. The Hague: Ministry of Health, Welfare and Sport, 2003
Kostenbeheersing in de zorg, Kamerstuk 24124 nr 39. The Hague: Tweede Kamer der Staten-Generaal, 1996
Vaststelling van de begroting van de uitgaven en de ontvangsten van het Ministerie van Volksgezondheid, Welzijn en Sport (XVI) voor het jaar 1997, Kamerstuk 25000 xvi nr 71. The Hague: Tweede Kamer der Staten-Generaal, 1997
Richtlijn voor farmaco-economisch onderzoek, Brief van de minister van Volksgezondheid, Welzijn en Sport aan de voorzitter van de Ziekenfondsraad. The Hague, Ministry of Health, Welfare and Sport, 1997
Riteco JA, de Heij UM, Van Luijn JCF, et al. Dutch guidelines for pharmacoeconomic research. Amstelveen: College voor Zorgverzekeringen, 1999
Oostenbrink JB, Koopmanschap MA, Rutten FFH. Standardisation of costs: the Dutch manual for costing in economic evaluations. Pharmacoeconomics 2002; 20 (7): 443–54
Oostenbrink JB, Bouwmans CAM, Koopmanschap MA, et al. Handleiding voor kostenonderzoek, methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Geactualiseerde versie 2004. Amstelveen: College voor Zorgverzekeringen, 2004
College voor zorgverzekeringen, CVZ Farmaco-economisch rapportpharmacoeconomic reports Thyrogen®. Amstelveen: College voor Zorgverzekeringen, 2003 [online]. Available from URL: http://www.cvz.nl/resources/cfh-0209thyrogenfertcm13-1771.pdf [Accessed 2004 Dec 17]
van Luijn JCF. CFH-Rapport risperidone depot (Risperdal Consta®) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2003 [online]. Available from URL: http://www.cvz.nUresources/cfh03-03risperidondepot-FEtcm132195.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport clopidogrel (Plavix®) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.cvz.nl/resources/cfh04-06%20clopidogrel%20FER_tcml3-6750.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport vardenafil (Levitra(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.cvz.nl/resources/cfh03-20%20vardenafil%20FER_tcml3-6806.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport eplerone (Inspra(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.evz.nl/resources/cfh04-26%20eplerenon%20FER_tcm13-9527.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport pimecrolimus (Plavix(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.cvz.nl/resources/cfh04-01%20pimecrolimus%20FER_tcm13-6084.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport Asi-colon (Oncovax(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.cvz.nl/resources/cfh04-oncovax%20FERtern13-6527.pdf [Accessed 2004 Dec 17]
Toenders WGM. CFH-Rapport omega-3-vetzuren (Omacor(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2004 [online]. Available from URL: http://www.evz.nl/ resources/cfh04-13-omega-3-vetzuren-FER_tcml3-7841.pdf [Accessed 2004 Dec 17]
van Luijn JCF. CFH-Rapport risperidone depot (Risperdal Consta(D) Bijlage FE rapport. Amstelveen: College voor Zorgverzekeringen, 2003 [online]. Available from URL: http://www.cvz.nUresources/cfh03-03risperidondepot-FEtcm132195.pdf [Accessed 2004 Dec 17]
Hill SR, Henry DA. Problems with the interpretation of pharmacoeconomic analyses: a review of submissions to the Australian Pharmaceutical Benefits Scheme. JAMA 2000; 283 (16: 2116–21
A proposal for methodological guidelines for economic evaluation of pharmaceuticals. Brussels: Belgian Society for Pharmacoepidemiology, 1995
Amtliche Verlautbarungen der osterreichischen Sozialversicherung; Verfahrensordnung (no 100) zur Herausgabe des Heilmittelverzeichnisses. Vienna: Der Hauptverband der 6sterreichischen Sozialversicherungstrager, 2003
Baltic guidelines for economic evaluation of pharmaceuticals. Health authorities from the Baltic countries, 2002 [online]. Available from URL: http://www.zea.gov.ly/does/new2002/doc24-l.pdf [Accessed 2004 Aug 8]
General guidelines for economic evaluation from the Pharmaceutical Benefits Board LNFAR. Solna: Swedish Pharmaceutical Benefits Board, 2003 [online]. Available from URL: http://www.Ifn.se/upload/English/ENGlfnar2003-eng.pdf [Accessed 2004 Aug 8]
Norwegian guidelines for pharmacoeconomic analysis in connection with applications for reimbursement. Oslo: Norwegian Medicines Control Authority, 2000 [online]. Available from URL: http://www.legemiddelverket.no/eng/reg/pharmacoeconomic-analysis-guidelines.htm [Accessed 2004 Aug 8]
Dickson M, Hurst J, Jacobzone S. OECD health working papers no. 4 survey of pharmacoeconomic assessment activity in eleven countries. France: OECD, 2003 [online]. Available from URL: http://www.oeed.org/dataoeed/27/25/2955828.pdf [Accessed 2004 Aug 8]
Bemkover JD, Corey R. Effective utilization of pharmacoeconomics for decision makers. Dis Manage Health Outcomes 2002; 10 (2): 75–80
Acknowledgements
Pharmerit received a grant from the Dutch Ministry of Health, Welfare and Sport to examine how to optimise the transparency and consistency of the reimbursement decision-making process, with a special focus on the use of pharmacoeconomic information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Oostenbruggen, M.F., Jansen, R.B., Mur, K. et al. Penny and pound wise. Pharmacoeconomics 23, 219–226 (2005). https://doi.org/10.2165/00019053-200523030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200523030-00003