Abstract
Abstract
Alzheimer’s disease is associated with a large cost burden, of which institutionalised care constitutes a major component. Therefore, the decision to move a patient from the community to institutionalised care is associated with a significant increase in direct costs. About three-quarters of patients with Alzheimer’s disease are admitted to a nursing home within 5 years of diagnosis. Unpaid or informal caregiver time is another large cost in Alzheimer’s disease, especially for patients cared for in the community; informal care can account for up to three-quarters of healthcare costs in non-institutionalised patients.
Several cholinesterase inhibitors, of which rivastigmine is one, are available for the treatment of patients with mild to moderate Alzheimer’s disease. By improving cognitive function and slowing the rate of cognitive decline, cholinesterase inhibitor therapy may reduce a significant part of the economic burden of the disease by delaying the move to institutionalised care. In the absence of prospective long term data which focus on pharmacoeconomic end-points, modelling techniques have been used to extrapolate clinical data available for some cholinesterase inhibitors, including rivastigmine.
Four economic analyses, based on a single model of cognitive decline, have been performed with rivastigmine from the perspective of the provider or society. All show that rivastigmine therapy (excluding drug-related costs) is associated with cost savings in patients with mild to moderate Alzheimer’s disease by delaying the time to institutionalisation. If the acquisition cost of the drug was factored in, the cost savings completely or partially offset treatment costs. The magnitude of the cost savings increased as the time horizon increased (up to 2 years). The largest savings were realised in patients with mild disease over a 2-year time-frame, suggesting that treatment should be initiated early from an economic viewpoint. Pharmacoeonomic data comparing different cholinesterase inhibitors are, as yet, unavailable.
Conclusion: Pharmacoeconomic analyses, based on modelled data excluding drug costs, indicate that rivastigmine completely or partially offsets the costs of treatment by delaying cognitive decline and the time to institutionalisation in patients with mild to moderate Alzheimer’s disease. From a societal perspective, cost savings are realised if the drug is introduced early in the disease. Additional benefits offered by rivastigmine on behavioural symptoms, which may reduce caregiver burden, have yet to be investigated froma pharmacoeconomic perspective.
Alzheimer’s Disease
Alzheimer’s disease is characterised by progressive loss of memory and cognitive function. It is the most common form of dementia with an average prevalence of about 5% (range 3.4 to 6.7%) in those aged 65 years or over. Between the ages of 65 and 90 years, the incidence of Alzheimer’s disease doubles for approximately every 5 years of age gained.
In addition to symptoms of cognitive decline and short term memory loss, some patients also experience psychiatric and behavioural disturbances as the disease progresses. The ability to perform basic activities of daily living, such as dressing, bathing and eating, also decreases once the disease becomes moderately severe. Severe dementia is characterised by complete dependence on caregivers.
Patients with Alzheimer’s disease are often cared for at home. Caregivers carry a considerable burden of emotional and physical strain. They are required to perform numerous tasks which can vary from assistance with high level tasks (such as managing finances) in the early stages of the disease to basic tasks (such as bathing) when the disease becomes more severe. There is a direct association between carer burden and behavioural symptoms. As the disease progresses and more supervision is required, the patient may be moved to institutionalised care. About three-quarters of patients with Alzheimer’s disease are admitted to a nursing home within 5 years of diagnosis and typically require care for up to 8 years.
Alzheimer’s disease is associated with a considerable cost burden. Estimates of the cost of care for a patient with Alzheimer’s disease range from approximately US dollars ($US) 17 000 to $US55 000 per annum (1995 values). Costs increase with increasing disease severity; the respective estimated annual costs for patients with mild, moderate and severe disease are $US18 408, $US30 096 and $US36 132 (1996 values).
Institutionalised care is the largest single component of healthcare costs in patients with more severe Alzheimer’s disease. Therefore, the decision to move a patient from the community to institutionalised care is associated with a significant increase in direct costs. Unpaid caregiver time (or informal care) also makes up a large proportion of costs, especially in patients with milder disease cared for at home. In non-institutionalised patients, unpaid care accounted for 50 to 74% of total costs for patients with Alzheimer’s disease.
Clinical Considerations in the Use of Rivastigmine
Symptomatic treatment for patients with Alzheimer’s disease is currently focused on augmentation of cholinergic neurotransmission. Several cholinesterase inhibitors are now available and all achieve a similar level of improvement in cognitive function. Differences between agents therefore relate not to their effects on cognition, but rather to differences in effects on behavioural symptoms and their tolerability, pharmacological and potential drug interaction profiles.
Rivastigmine, like other cholinesterase inhibitors, produces modest improvements in cognitive function. Two pivotal trials showed that rivastigmine, at an oral dosage of 6 to 12 mg/day, significantly improved measures of cognitive function after 26 weeks of treatment versus placebo in patients with mild to moderate Alzheimer’s disease. Caregiver-rated activities of daily living also improved in significantly more patients receiving rivastigmine in both trials (25 and 29% vs 15 and 19% with placebo). Rivastigmine also was superior to placebo on the Clinician’s Interview Based Impression of Change Plus (CIBIC-plus)] scale, which measures global functioning. In addition, noncomparative clinical trials show that rivastigmine offers long term benefits on behavioural symptoms in patients with Alzheimer’s disease.
As with other cholinesterase inhibitors, the adverse events most commonly associated with rivastigmine are cholinergic in nature. These include nausea, vomiting, diarrhoea and anorexia, and were reported in 14 to 50% of patients receiving rivastigmine 6 to 12 mg/day compared with 2 to 11% of placebo recipients in clinical trials.Most events are of mild to moderate intensity, dose-related and of limited duration. About one-quarter of patients receiving rivastigmine 6 to 12 mg/day discontinue treatment because of adverse events. No clinically relevant changes in laboratory or vital signs (including hepatic enzymes) are observed.
Pharmacoeconomic Analyses
In producing modest improvements and, more importantly, slowing the decline of cognitive function, cholinesterase inhibitors may have the potential to decrease a significant part of the economic burden of Alzheimer’s disease by delaying the time to institutionalisation. Long term prospective data are required to quantify and assess the costs of these effects but are currently unavailable. In the interim, an alternative option is to use modelling techniques on pivotal clinical trial data to estimate the long term cost implications of treatment. Such an approach has been used with rivastigmine and some other cholinesterase inhibitors.
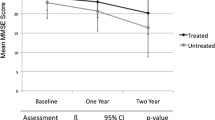
A model based on pivotal clinical trial data in patients with mild to moderate Alzheimer’s disease has been developed to estimate the delay in cognitive decline offered by rivastigmine over placebo. This model forms the basis for 4 pharmacoeconomic analyses on rivastigmine.Mini-Mental State Examination scores are used as a measure of disease progression or cognitive decline, and an accelerated life model is used to extrapolate the data beyond the end of the clinical trials (1 and 2 years). Each analysis assessed slightly different components of cost, although all costs were derived from recent cost-of-illness data from the US, UK or Canada.
The analyses show that treatment with rivastigmine 6 to 12 mg/day is associated with cost savings (exclusive of drug-related costs) in patients with mild to moderate Alzheimer’s disease. The magnitude of the savings increased as the time horizon increased. For example, in the most recent US analysis, savings achieved per patient with mild disease were $US83, $US683 and $US4768 after 6 months, 1 and 2 years, respectively.
When disease severity was considered, the greatest savings were realised in those with mild disease over a 2-year time-frame. However, over a time-frame of less than 2 years, greater costs savings were observed in those with moderate disease because institutionalisation is more likely in this patient group.







Similar content being viewed by others
References
Baldereschi M, di Carlo A, Amaducci L. Epidemiology of dementias. Drugs Today 1998 Sep; 34: 747–58
Mayeux R, Sano M. Treatment of Alzheimer’s disease. N Engl J Med 1999 Nov 25; 341: 1670–9
Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology 1998 Sep; 51: 728–33
Meek PD, McKeithan K, Schumock GT. Economic considerations in Alzheimer’s disease. Pharmacotherapy 1998 Mar–Apr; 18 (2 Pt 2): 68–73, discussion 79-82
Wimo A, Winblad B, Grafstrom M. The social consequences for families with Alzheimer’s disease patients: potential impact of new drug treatment. Int J Geriatr Psychiatry 1999 May; 14: 338–47
Mohide EA. Informal care of community-dwelling patients with Alzheimer’s disease: focus on the family caregiver. Neurology 1993 Aug; 43 Suppl. 4: S16–9
Coon DW, Edgerly ES. The personal and social consequences of Alzheimer disease. Genet Test 1999; 3: 29–36
Hope T, Keene J, Fairburn G, et al. Natural history of behavioural changes and psychiatric symptoms in Alzheimer’s disease. Br J Psychiatry 1999; 174: 39–44
Winblad B, Wimo A, Mobius HJ, et al. Severe dementia: a common condition entailing high costs at individual and societal levels [editorial]. Int J Geriatr Psychiatry 1999 Nov; 14: 911–4
Trabucchi M. An economic perspective on Alzheimer’s disease. J Geriatr Psychiatry Neurol 1999 Spring; 12: 29–38
Francis PT, Palmer AM, Snape M, et al. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 1999 Feb; 66: 137–47
Winblad B, Wimo A. Assessing the societal impact of acetylcholinesterase inhibitor therapies. Alzheimer Dis Assoc Disord 1999 Nov; 13 Suppl. 2: S9–19
Schneider J, Murray J, Banerjee S, et al. EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer’s disease: I-Factors associated with carer burden. Int J Geriatr Psychiatry 1999 Aug; 14: 651–61
Rice DP, Fox PJ, Max W, et al. The economic burden of Alzheimer’s disease care. Health Aff (Millwood) 1993 Summer; 12 (2): 164–76
Souêtre E, Thwaites RM, Yeardley HL. Economic impact of Alzheimer’s disease in the United Kingdom: cost of care and disease severity for non-institutionalised patients with Alzheimer’s disease. Br J Psychiatry 1999 Jan; 174: 51–5
Welch HG, Walsh JS, Larson EB. The cost of institutional care in Alzheimer’s disease: nursing home and hospital use in a prospective cohort. J Am Geriatr Soc 1992 Mar; 40: 221–4
Heyman A, Peterson B, Fillenbaum G, et al. Predictors of time to institutionalization of patients with Alzheimer’s disease: the CERAD experience, part XVII. Neurology 1997 May; 48: 1304–9
Brodaty H, McGilchrist C, Harris L, et al. Time until institutionalization and death in patients with dementia: role of caregiver training and risk factors. Arch Neurol 1993 Jun; 50: 643–50
Heyman A, Wilkinson WE, Hurwitz BJ, et al. Early-onset Alzheimer’s disease: clinical predictors of institutionalization and death. Neurology 1987 Jun; 37: 980–4
Knopman DS, Kitto J, Deinard S, et al. Longitudinal study of death and institutionalization in patients with primary degenerative dementia. J Am Geriatr Soc 1988 Feb; 36 (2): 108–12
Haupt M, Kurz A. Predictors of nursing home placement in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1993; 8: 741–6
Colerick EJ, George LK. Predictors of institutionalization among caregivers of patients with Alzheimer’s disease. J Am Geriatr Soc 1986 Jul; 34 (7): 493–8
Knapp M, Wilkinson D, Wigglesworth R. The economic consequences of Alzheimer’s disease in the context of new drug developments. Int J Geriatr Psychiatry 1998 Aug; 13: 531–43
Winblad B, Hill S, Beermann B, et al. Issues in the economic evaluation of treatment for dementia. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alz Dis Assoc Disord 1997; 11 Suppl. 3: 39–45
Ernst RL, Hay JW. Economic research on Alzheimer disease: a review of the literature. Alz Dis Assoc Disord 1997; 11 Suppl. 6: 135–45
Leon J, Cheng C-K, Neumann PJ. Alzheimer’s disease care: costs and potential savings. Health Aff 1998 Nov-Dec; 17: 206–16
Hux MJ, O’Brien BJ, Iskedjian M, et al. Relation between severity of Alzheimer’s disease and costs of caring. Can Med Assoc J 1998 Sep 8; 159: 457–65
Whitehouse PJ, Winblad B, Shostak D, et al. First International Pharmacoeconomic Conference on Alzheimer’s Disease: report and summary. Alzheimer Dis Assoc Disord 1998 Dec; 12: 266–80
Hay JW, Sano M, Whitehouse PJ. The costs and social burdens of Alzheimer disease: what can and should be done? Alzheimer Dis Assoc Disord 1997 Dec; 11: 181–3
Schumock GT. Economic considerations in the treatment and management of Alzheimer’s disease. Am J Health Syst Pharm 1998 Nov 1; 55 Suppl. 2: S17–21
Ernst RL, Hay JW. The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health 1994 Aug; 84 (8): 1261–4
Giacobini E. Invited review: cholinesterase inhibitors for Alzheimer’s disease therapy: from tacrine to future applications. Neurochem Int 1998 May-Jun; 32: 413–9
VanDenBerg CM, Kazmi Y, Jann MW. Cholinesterase inhibitors for the treatment of Alzheimer’s disease in the elderly. Drugs Aging 2000 Feb; 16: 123–38
Nordberg A, Svensson A-L. Cholinesterase inhibitors in the treatment of Alzheimer’s disease: a comparison of tolerability and pharmacology [published erratum appears in Drug Saf 1999 Feb; 20 (2): 146]. Drug Saf 1998 Dec; 19: 465–80
Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000 Jan; 157: 4–15
Krall WJ, Sramek JJ, Cutler NR. Cholinesterase inhibitors: a therapeutic strategy for Alzheimer disease. Ann Pharmacother 1999 Apr; 33: 441–50
Jann MW. Pharmacology and clinical efficacy of cholinesterase inhibitors. Am J Health Syst Pharm 1998 Nov 1; 55 Suppl. 2: S22–5
Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 2000 Jun; 54: 2269–76
Weinstock M. Selectivity of cholinesterase inhibition: clinical implications for the treatment of Alzheimer’s disease. CNS Drugs 1999 Oct; 12 (4): 307–23
Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs 2000 Nov; 60 (5): 1095–122
Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised control trial. BMJ 2000 Dec; 321: 1145–449
Cummings J, Anand R, Koumaras B, et al. Rivastigmine provides behavioral benefits to Alzheimer’s disease patients residing in a nursing home: findings from a 26-week trial [abstract]. Neurology 2000 Apr 11; 54 Suppl. 3: A468–469
Cummings JL, Anand R, Koumaras B, et al. Behavioral benefits in Alzheimer’s disease patients residing in a nursing home following 52 weeks of rivastigmine treatment [abstract no. NR574]. American Psychiatric Association 2000 Annual Meeting; 2000 May 13–18; Chicago, 212–3
Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial [see comments]. BMJ 1999 Mar 6; 318: 633–8
Corey-Bloom J, Anand R, Veach J, et al. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998 Jun; 1: 55–65
Anand R, Messina J, Hartman R. Dose-response effect of rivastigmine in the treatment of Alzheimer’s disease. Int J Geriatr Psychopharmacol 2000; 2: 68–72
Farlow M, Messina J, Anand R. Long-term cognitive benefits associated with the use of rivastigmine in the treatment of Alzheimer’s disease: results following two years of treatment [abstract no. P396]. J Am Geriatr Soc 2000 Aug; 48 (8): 108
Rösler M, Retz W, Retz-Junginger P, et al. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol 1998/1999; 11: 211–6
McKeith I, Del Ser T, Spano PF, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356: 2031–6
Babic T, Banfic L, Papa J, et al. Spontaneous rupture of oesophagus (Boerhaave’s syndrome) related to rivastigmine [letter]. Age Aging 2000 Jul; 29 (4): 370–1
Novartis Pharmaceuticals Corporation. Exelon (rivastigmine tartrate) capsules prescribing information. Apr 2000
Cunningham SC. Change to Exelon prescribing information. Available from: URL: http://www.fda.gov/medwatch/safety/2001/exelon.htm [Accessed 2000 Feb 8]
Rösler M, B303 Exelon Study Group. Effectiveness of rivastigmine in Alzheimer’s disease: reply [letter]. BMJ 1999 Sep 4; 319: 641
Bentham P, Gray R, Sellwood E, et al. Effectiveness of rivastigmine in Alzheimer’s disease: improvements in functional ability remain unestablished [letter]. BMJ 1999 Sep 4; 319: 640–1
Bentham P, Gray R, Raftery J. Effectiveness of rivastigmine in Alzheimer’s disease: participation in trials should be based on clinical uncertainty, not enforcement: authors’ reply [letter]. BMJ 2000 Feb 19; 320: 512
Foster RH, Plosker GL. Donepezil: pharmacoeconomic implications of therapy. Pharmacoeconomics 1999 Jul; 16 (1): 99–114
Max W. Drug treatments for Alzheimer’s disease: shifting the burden of care. CNS Drugs 1999 May; 11: 363–72
Fenn P, Gray A. Estimating long term cost savings from treatment of Alzheimer’s disease: a modelling approach. Pharmacoeconomics 1999 Aug; 16 (2): 165–74
Brooks E, Deal L. The effect of rivastigmine on the direct and indirect costs of Alzheimer’s disease [abstract no. PMH5 + poster]. Value Health 2000 Mar/Apr; 3 (2): 79
Ernst RL, Hay JW, Fenn C, et al. Cognitive function and the costs of Alzheimer disease: an exploratory study. Arch Neurol 1997 Jun; 54: 687–93
Gray A, Fenn P. Alzheimer’s disease: the burden of the illness in England. Health Trend 1993; 25 (1): 31–7
Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther 2000 Apr; 22: 439–51
Hauber AB, Gnanasakthy A, Snyder EH, et al. Potential savings in the cost of caring for Alzheimer’s disease: patient treatment with rivastigmine. Pharmacoeconomics 2000 Apr; 17: 351–60
Mends P, Snyder E, Gause D, et al. Cost savings from decreased utilization of antipsychotic drugs associated with rivastigmine use in long-term care facilities [abstract no. 760]. Neurobiol Aging 2000; 21 Suppl. 1: S167
Knopman D, Schneider L, Davis K, et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality. Tacrine Study Group. Neurology 1996 Jul; 47 (1): 166–77
Small GW, Donohue JA, Brooks RL. An economic evaluation of donepezil in the treatment of Alzheimer’s disease. Clin Ther 1998 Jul–Aug; 20 (4): 838–50
Whitehouse PJ. Cholinesterase inhibitors in Alzheimer’s disease: are they worth the cost? CNS Drugs 1999 Mar; 11: 167–73
Johnson N, Davis T, Bosanquet N. The epidemic of Alzheimer’s disease: how can we manage the costs? Pharmacoeconomics 2000 Sep; 18 (3): 215–23
Data on file, Novartis Pharmaceuticals Corporation, 2000 Apr
Aricept better tolerated than Exelon, claim Eisai/Pfizer. Scrip 2000 Oct 11; 2582: 22
Max W. The cost of Alzheimer’s disease: will drug treatment ease the burden? Pharmacoeconomics 1996 Jan; 9 (1): 5–10
Marin DB, Snyder EH, Hauber AB, et al. Impact of rivastigmine on costs and time spent caring for Alzheimer’s disease patients [abstract no. 1301]. Qual Life Res 2000; 9 (3): 271
Bianchetti A, Frisoni GB, Ghisia KM, et al. Clinical predictors of the indirect costs of Alzheimer disease [letter; comment]. Arch Neurol 1998 Jan; 55: 130–1
Ernst RL, Hay JW, Fenn C, et al. Clinical predictors of the indirect costs of Alzheimer disease: reply [letter]. Arch Neurol 1998 Jan; 55: 131
Beck CK, Cody M, Zhang M. A multidisciplinary team approach to managing Alzheimer’s disease. Pharmacotherapy 1998 Mar–Apr; 18 (2 Pt 2): 33–42, discussion 79-82
Mittelman MS, Ferris SH, Schulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. JAMA 1996 Dec 4; 276 (21): 1725–31
Fox PJ. Service use and cost outcomes for persons with Alzheimer disease. Alz Dis Assoc Disord 1997; 11 Suppl. 6: 125–34
Vetter P, Steiner O, Kraus S, et al. Factors affecting the utilization of homecare supports by caregiving relatives of Alzheimer patients. Dement Geriatr Cogn Disord 1998 Mar–Apr; 9: 111–6
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: H. Brodaty, Academic Department for Old Age Psychiatry, University of New South Wales, Prince of Wales Hospital, Sydney, Australia; J.L. Cummings, Department of Neurology, UCLA Alzheimer’s Disease Center, Los Angeles, California, USA; W.B. Dalziel, Regional Geriatric Assessment Program, Ottawa Civic Hospital, Ottawa, Ontario, Canada; P.J. Fox, Institute for Health & Aging, Department of Social and Behavioral Sciences, University of California, San Francisco, California, USA; W. Retz, Study Group Gerontopsychiatry, Department of Psychiatry, University of Saarland, Homburg/Saar, Germany; A. Wimo, The Research Unit of Primary Health Care in Noranstig, Bergasjö, Sweden; B. Winblad, Department of Clinical Neuroscience, Occupational Therapy and Elderly Care, Division of Geriatric Medicine, Karolinska Institute, Stockholm, Sweden.
Data Selection
Sources: Medical literature published in any language since 1966 on rivastigmine, identified using Medline supplemented by AdisBase (a proprietary database of Adis International, Auckland, New Zealand). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘rivastigmine’ or ‘Alzheimers-disease’ and (‘health-economics’ or ‘pharmacoepidemiology’ or ‘prescribing’ or ‘hospitalisation’ or ‘formularies’ or ‘drug-utilisation’ or ‘meta-analysis’ or ‘therapeutic-substitution’ or ‘epidemiology’), or ‘rivastigmine’ and ‘Alzheimers-disease’. Medline search terms were ‘rivastigmine’ or ‘Alzheimer-disease’ and (‘economics’ or ‘health-policy’ or ‘quality-of-life’ or ‘models-statistical’ or ‘health-planning’ or ‘epidemiology’ or ‘guideline in pt’ or ‘practice-guidelines in pt’. Searches were last updated 12 Feb 2001.
Selection: Clinical trials and economic analyses of rivastigmine in patients with Alzheimer’s disease. Relevant background data on epidemiology and cost of illness are also included.
Index terms: Rivastigmine, Alzheimer’s disease, pharmacoeconomics, cost analyses, therapeutic use, cholinesterase inhibitors.
Rights and permissions
About this article
Cite this article
Lamb, H.M., Goa, K.L. Rivastigmine. Pharmacoeconomics 19, 303–318 (2001). https://doi.org/10.2165/00019053-200119030-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200119030-00008