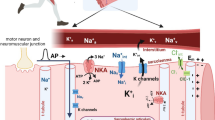
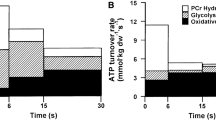
Abstract
This article critically discusses whether accumulation of lactic acid, or in reality lactate and/or hydrogen (H+) ions, is a major cause of skeletal muscle fatigue, i.e. decline of muscle force or power output leading to impaired exercise performance. There exists a long history of studies on the effects of increased lactate/H+ concentrations in muscle or plasma on contractile performance of skeletal muscle. Evidence suggesting that lactate/H+ is a culprit has been based on correlation-type studies, which reveal close temporal relationships between intramuscular lactate or H+ accumulation and the decline of force during fatiguing stimulation in frog, rodent or human muscle. In addition, an induced acidosis can impair muscle contractility in non-fatigued humans or in isolated muscle preparations, and several mechanisms to explain such effects have been provided. However, a number of recent high-profile papers have seriously challenged the ‘lactic acid hypothesis’. In the 1990s, these findings mainly involved diminished negative effects of an induced acidosis in skinned or intact muscle fibres, at higher more physiological experimental temperatures. In the early 2000s, it was conclusively shown that lactate has little detrimental effect on mechanically skinned fibres activated by artificial stimulation. Perhaps more remarkably, there are now several reports of protective effects of lactate exposure or induced acidosis on potassium-depressed muscle contractions in isolated rodent muscles. In addition, sodium-lactate exposure can attenuate severe fatigue in rat muscle stimulated in situ, and sodium lactate ingestion can increase time to exhaustion during sprinting in humans. Taken together, these latest findings have led to the idea that lactate/ H+ is ergogenic during exercise.
It should not be taken as fact that lactic acid is the deviant that impairs exercise performance. Experiments on isolated muscle suggest that acidosis has little detrimental effect or may even improve muscle performance during high-intensity exercise. In contrast, induced acidosis can exacerbate fatigue during whole-body dynamic exercise and alkalosis can improve exercise performance in events lasting 1–10 minutes. To reconcile the findings from isolated muscle fibres through to whole-body exercise, it is hypothesised that a severe plasma acidosis in humans might impair exercise performance by causing a reduced CNS drive to muscle.




Similar content being viewed by others
References
Cady EB, Jones DA, Lynn J, et al. Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 1989; 418: 311–325
Cairns SP, Buller SJ, Loiselle DS, et al. Changes of action potentials and force at lowered [Na+]o in mouse skeletal muscle: implications for fatigue. Am J Physiol 2003; 285: C1529–C1536
Cairns SP, Hing WA, Slack JR, et al. Role of extracellular [Ca2+] in fatigue of isolated mammalian skeletal muscle. J Appl Physiol 1998; 84: 1395–1406
Cairns SP, Ruzhynsky V, Renaud JM. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am J Physiol 2004; 287: C762–C770
Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 1994; 74: 49–94
Green HJ. Mechanisms of muscle fatigue in intense exercise. J Sports Sci 1997; 15: 247–256
Lindinger MI, McKelvie RS, Heigenhauser GJ. K+ and Lac-distribution in humans during and after high-intensity exercise: role in fatigue attenuation. J Appl Physiol 1995; 78: 765–777
Cairns SP, Knicker AJ, Thompson MW, et al. Evaluation of models used to study neuromuscular fatigue. Exerc Sport Sci Rev 2005; 33 (1): 9–16
Allen DG, Westerblad H, Lännergren J. The role of intracellular acidosis in muscle fatigue. Adv Exp Med Biol 1995; 384: 57–68
Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol 2004; 558: 5–30
Heigenhauser GJF, Jones NL. Bicarbonate loading. In: Lamb DR, Williams MH, editors. Vol. 4. Perspectives in exercise science and sports medicine. Carmel (MI): Cooper Publishing Group, 1991: 183–207
Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand Suppl 1986; 556: 83–91
Fletcher WM, Hopkins G. Lactic acid in amphibian muscle. J Physiol 1907; 35: 247–309
Hill AV, Kupalov P. Anaerobic and aerobic activity in isolated muscle. Proc R Soc Lond B 1929; 105: 313–322
Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med 1923; 16: 135–171
Bassett DR. Scientific contributions of A.V. Hill: exercise physiology pioneer. J Appl Physiol 2002; 93: 1567–1582
Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced acidosis. Am J Physiol 2004; 287: R502–R516
Robergs RA, Ghiasvand F, Parker D. Lingering construct of lactic acidosis. Am J Physiol 2005; 289: R904–R910
Lindinger MI, Kowalchuk JM, Heigenhauser GJF. Applying physiochemical principles to skeletal muscle acid-base status. Am J Physiol 2005; 289: R891–R894
Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature 1978; 274: 861–866
Hermansen L, Osnes JB. Blood and muscle pH after maximal exercise in man. J Appl Physiol 1972; 32: 304–308
Sahlin K, Alvestrand A, Brandt R, et al. Intracellular pH and bicarbonate concentration in human muscle during recovery from exercise. J Appl Physiol 1978; 45: 474–480
Sahlin K, Harris RC, Nylind B, et al. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflügers Arch 1976; 367: 143–149
Brooks GA. Lactate doesn’t necessarily cause fatigue: why are we surprised? J Physiol 2001; 536: 1
Lydiard A, Gilmour G. Running with Lydiard. Auckland: Hod-der & Stroughton, 1983
Spriet LL, Söderlund K, Bergström M, et al. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J Appl Physiol 1987; 62: 616–621
Juel C. Lactate-proton cotransport in skeletal muscle. Physiol Rev 1997; 77: 321–358
Cairns SP, Westerblad H, Allen DG. Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol 1993; 464: 561–574
Donaldson SKB, Hermansen L. Differential, direct effects of H+ on Ca2+-activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflügers Arch 1978; 376: 55–65
Leitch SP, Paterson DJ. Interactive effects of K+, acidosis, and catecholamines on isolated rabbit heart: implications for exercise. J Appl Physiol 1994; 77: 1164–1171
Paterson DJ. Antiarrhythmic mechanisms during exercise. J Appl Physiol 1996; 80: 1853–1862
Fitts RH, Holloszy JO. Lactate and contractile force in frog muscle during development of fatigue and recovery. Am J Physiol 1976; 231: 430–433
Troup JP, Metzger JM, Fitts RH. Effect of high-intensity exercise training on functional capacity of limb skeletal muscle. J Appl Physiol 1986; 60: 1743–1751
Spriet LL, Söderlund K, Bergström M, et al. Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol 1987; 62: 611–615
Adams GR, Fisher MJ, Meyer RA. Hypercapnic acidosis and increased H2PO4- concentration do not decrease force in cat skeletal muscle. Am J Physiol 1991; 260: C805–C812
Meyer RA, Adams GR, Fisher MJ, et al. Effect of decreased pH on force and phosphocreatine in mammalian skeletal muscle. Can J Physiol Pharmacol 1991; 69: 305–310
Renaud JM. The effect of lactate on intracellular pH and force recovery of fatigued sartorius muscles of the frog, Rana pipiens. J Physiol 1989; 416: 31–47
Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 1992; 449: 49–71
Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J 1988; 53: 935–946
Dutka TL, Lamb GD. Effect of lactate on depolarization-induced Ca2+ release in mechanically skinned skeletal muscle fibers. Am J Physiol 2000; 278: C517–C525
Posterino GS, Fryer MW. Effects of high myoplasmic L-lactate concentration on E-C coupling in mammalian skeletal muscle. J Appl Physiol 2000; 89: 517–528
Favero TG, Zable AC, Bowman MB, et al. Metabolic end products inhibit sarcoplasmic reticulum Ca2+ release and [3H]ryanodine binding. J Appl Physiol 1995; 78: 1665–1672
Booth J, McKenna MJ, Ruell PA, et al. Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. J Appl Physiol 1997; 83: 511–521
Stephens TJ, McKenna MJ, Canny BJ, et al. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc 2002; 34: 614–621
Bogdanis GC, Nevill ME, Lakomy HKA, et al. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol Scand 1998; 163: 261–272
Nevill ME, Boobis LH, Brooks S, et al. Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol 1989; 67: 2376–2382
Nielsen JJ, Mohr M, Klarskov C, et al. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol 2003; 554 (Pt 3): 857–870
Achten E, Van Cauteren M, Willem R, et al. 31P-NMR spectroscopy and the metabolic properties of different muscle fibers. J Appl Physiol 1990; 68: 644–649
Mannion AF, Jakeman PM, Willan PLT. Skeletal muscle buffer value, fibre type distribution and high intensity exercise performance in man. Exp Physiol 1995; 80: 89–101
DeGroot M, Massie BM, Boska M, et al. Dissociation of [H+] from fatigue in human muscle detected by high time resolution 31P-NMR. Muscle Nerve 1993; 16: 91–98
Chasiotis D, Hultman E, Sahlin K. Acidotic depression of cyclic AMP accumulation and phosphorylase b to a transformation in skeletal muscle of man. J Physiol 1982; 335: 197–204
Costill DL, Barnett A, Sharp R, et al. Leg muscle pH following sprint running. Med Sci Sports Exerc 1983; 15: 325–329
Mainwood GW, Renaud JM. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol 1985; 63: 403–416
Westerblad H, Lännergren J. The relation between force and intracellular pH in fatigued, single Xenopus muscle fibres. Acta Physiol Scand 1988; 133: 83–89
Bruton JD, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol 1998; 85: 478–483
Chin ER, Allen DG. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol 1998; 512: 831–840
Nielsen HB, Bredmose PP, Strømstad M, et al. Bicarbonate attenuates arterial desaturation during maximal exercise in humans. J Appl Physiol 2002; 93: 724–731
Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol 2001; 537: 993–998
Spriet LL, Matsos CG, Peters SJ, et al. Effects of acidosis on rat metabolism and performance during heavy exercise. Am J Physiol 1985; 248: C337–C347
Cooke R, Franks K, Luciani GB, et al. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol 1988; 395: 77–97
Nosek TM, Fender KY, Godt RE. It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science 1987; 236: 191–193
Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol 1994; 478: 331–339
Hultman E, Del Canale S, Sjoholm H. Effect of induced metabolic acidosis on intracellular pH, buffer capacity and contraction force of human skeletal muscle. Clin Sci 1985; 69: 505–510
Kowalchuk JM, Heigenhauser GJF, Jones NL. Effect of pH on metabolic and cardiorespiratory responses during progressive exercise. J Appl Physiol 1984; 57: 1558–1563
Sutton JR, Jones NL, Toews CJ. Effect of pH on muscle glycolysis during exercise. Clin Sci 1981; 61: 331–338
McCartney N, Heigenhauser GJF, Jones NL. Effects of pH on maximal power output and fatigue during short-term dynamic exercise. J Appl Physiol 1983; 55: 225–229
Balog EM, Fitts RH. Effects of depolarization and low intracellular pH on charge movement currents of frog skeletal muscle fibers. J Appl Physiol 2001; 90: 228–234
Rousseau E, Pinkos E. pH modulates conducting and gating behaviour of single calcium release channels. Pflügers Arch 1990; 415: 645–647
Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem 1966; 241: 4110–4114
Bangsbo J, Madsen K, Kiens B, et al. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol 1996; 495: 587–596
Linderman J, Fahey TD. Sodium bicarbonate ingestion and exercise performance: an update. Sports Med 1991; 11: 71–77
Pate E, Bhimani M, Franks-Skiba K, et al. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 1995; 486: 689–694
Wiseman RW, Beck TW, Chase PB. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol 1996; 271: C878–C886
Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol 1997; 500: 193–204
Davies NW. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature 1990; 343: 375–377
Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci 2002; 17: 17–21
Sprague P, Mann RV. The effects of muscular fatigue on the kinetics of sprint running. Res Q Exerc Sport 1983; 54: 60–66
Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett 1998; 257: 109–112
Van Montfoort MCE, Van Dieren L, Hopkins WG, et al. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc 2004; 36: 1239–1243
Karelis AD, Marcil M, Péronnet F, et al. Effect of lactate infusion on M-wave characteristics and force in the rat plantaris muscle during repeated stimulation in situ. J Appl Physiol 2004; 96: 2133–2138
Sahlin K, Katz A, Henriksson J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem J 1987; 245: 551–556
Lewis SF, Haller RG. The pathophysiology of McArdle’s disease: clues to regulation in exercise and fatigue. J Appl Physiol 1986; 61: 391–401
Renaud JM, Light P. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens: implication for fatigue in vivo. Can J Physiol Pharmacol 1992; 70: 1236–1246
Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 2001; 536 (Pt 1): 161–166
Kristiensen M, Albertsen J, Rentsch M, et al. Lactate and force production in skeletal muscle. J Physiol 2005; 562 (Pt 2): 521–526
Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and fh-agonist. J Physiol 2003; 551: 277–286
Pedersen TH, De Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol 2005; 125: 237–246
Pedersen TH, Nielsen OB, Lamb GD, et al. Intracellular acidosis enhances the excitability of working muscle. Science 2004; 305: 1144–1147
van Emst M, Klarenbeek S, Schot A, et al. Reducing chloride conductance prevents hyperkalaemia-induced loss of twitch force in rat slow-twitch muscle. J Physiol 2004; 561: 169–181
Allen DG, Westerblad H. Lactic acid — the latest performance-enhancing drug. Science 2004; 305: 1112–1113
Raymer GH, Marsh GD, Kowalchuk JM, et al. Metabolic effects of induced alkalosis during progressive forearm exercise to fatigue. J Appl Physiol 2004; 96: 2050–2056
Verbitsky O, Mizrahi J, Levin M, et al. Effect of ingested sodium bicarbonate on muscle force, fatigue, and recovery. J Appl Physiol 1997; 83: 333–337
Spriet LL, Lindinger MI, Heigenhauser GJF, et al. Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol 1986; 251: R833–R839
Swank A, Robertson RJ. Effect of induced alkalosis on perception of exertion during intermittent exercise. J Appl Physiol 1989; 67: 1862–1867
Dousset E, Steinberg JG, Balon N, et al. Effects of acute hypoxemia on force and surface EMG during sustained handgrip. Muscle Nerve 2001; 24: 364–371
Garner SH, Sutton JR, Burse RL, et al. Operation Everest II: neuromuscular performance under conditions of extreme simulated altitude. J Appl Physiol 1990; 68: 1167–1172
Acknowledgements
The author gratefully thanks Drs Will Hopkins, Graham Lamb, Mike Lindinger and Chris Whatman for helpful discussion and comments on the manuscript. No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cairns, S.P. Lactic Acid and Exercise Performance. Sports Med 36, 279–291 (2006). https://doi.org/10.2165/00007256-200636040-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200636040-00001