Abstract
Ceftaroline is a broad-spectrum cephalosporin currently under clinical investigation for the treatment of complicated skin and skin-structure infections (cSSSI), including those caused by meticillin-resistant Staphylococcus aureus (MRSA), and community-acquired pneumonia (CAP). Ceftaroline has the ability to bind to penicillin-binding protein (PBP)2a, an MRSA-specific PBP that has low affinity for most other β-lactam antibacterials. The high binding affinity of ceftaroline to PBP2a (median inhibitory concentration 0.90 μg/mL) correlates well with its low minimum inhibitory concentration for MRSA. Ceftaroline is active in vitro against Gram-positive cocci, including MRSA, meticillin-resistant Staphylococcus epidermidis, penicillin-resistant Streptococcus pneumoniae and vancomycin-resistant Enterococcus faecalis (not E. faecium). The broad-spectrum activity of ceftaroline includes many Gram-negative pathogens but does not extend to extended-spectrum β-lactamase-producing or AmpC-derepressed Enterobacteriaceae or most nonfermentative Gram-negative bacilli. Ceftaroline demonstrates limited activity against anaerobes such as Bacteroides fragilis and non-fragilis Bacteroides spp. Limited data show that ceftaroline has a low propensity to select for resistant subpopulations.
Ceftaroline fosamil (prodrug) is rapidly converted by plasma phosphatases to active ceftaroline. For multiple intravenous doses of 600 mg given over 1 h every 12 hours for 14 days, the maximum plasma concentration was 19.0 μg/mL and 21.0 μg/mL for first and last dose, respectively. Ceftaroline has a volume of distribution of 0.37 L/kg (28.3 L), low protein binding (<20%) and a serum half-life of 2.6 hours. No drug accumulation occurs with multiple doses and elimination occurs primarily through renal excretion (49.6%). Based on Monte Carlo simulations, dosage adjustment is recommended for patients with moderate renal impairment (creatinine clearance 30–50 mL/min); no adjustment is needed for mild renal impairment.
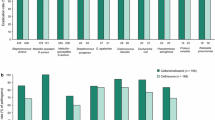
Currently, limited clinical trial data are available for ceftaroline. A phase II study randomized 100 patients with cSSSI to intravenous ceftaroline 600 mg every 12 hours or intravenous vancomycin 1 g every 12 hours with or without intravenous aztreonam 1 g every 8 hours (standard therapy) for 7–14 days. Clinical cure rates were 96.7% for ceftaroline compared with 88.9% for standard therapy. Adverse events were similar between groups and generally mild in nature. In a phase III trial, 702 patients with cSSSI were randomized to ceftaroline 600 mg or vancomycin 1 g plus aztreonam 1 g, each administered intravenously every 12 hours for 5–14 days. Ceftaroline was noninferior to vancomycin plus aztreonam in treating cSSSI caused by both Gram-positive and -negative pathogens. Adverse event rates were similar between groups.
Ceftaroline is well tolerated, which is consistent with the good safety and tolerability profile of the cephalosporin class. In summary, ceftaroline is a promising treatment for cSSSI and CAP, and has potential to be used as monotherapy for polymicrobial infections because of its broad-spectrum activity. Further clinical studies are needed to determine the efficacy and safety of ceftaroline, and to define its role in patient care.









Similar content being viewed by others
References
Centers for Disease Control and Prevention. S. aureus and MRSA Surveillance Summary 2007. Department of Health and Human Services, Centres for Disease Control and Prevention October 17, 2007 [online]. Available from URL: http://www.cdc.gov/ncidod/dhqp/ar_mrsa_surveillanceFS.html [Accessed 2008 May 22]
Klevens RM, Edwards JR, Tenover FC, et al. National Nosocomial Infections Surveillance System. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis 2006 Feb; 42(3): 389–91
Conly J. Antimicrobial resistance in Canada. CMAJ 2002 Oct 15; 167(8): 885–91
CNISP PCSIN. Surveillance for methicillin-resistant Staphylococcus aureus (MRSA) 2006 results. Canadian Nosocomial Infection Surveillance Program (CNISP), Public Health Agency of Canada 2006 [online]. Available from URL: http://www.phac-aspc.gc.ca/nois-sinp/projects/pdf/mrsa_report2006-eng.pdf [Accessed 2008 May 26]
Canadian Nosocomial Infection Surveillance Program. Surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospitals: a report update from the Canadian Nosocomial Infection Surveillance Program. Can Commun Dis Rep 2005 Feb; 31(3): 33–9
Tiemersma EW, Bronzwaer SL, Lyytikäinen O, et al. European Antimicrobial Resistance Surveillance System Participants. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis 2004 Sep; 10(9): 1627–34
Sievert DM, Rudrik JT, Patel JB, et al. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008 Mar; 46(5): 668–74
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009 Jan; 66(1): 82–98
Vardakas KZ, Ntziora F, Falagas ME. Linezolid: effectiveness and safety for approved and off-label indications. Expert Opin Pharmacother 2007 Oct; 8(14): 2381–400
Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother 2002 Aug; 46(8): 2723–6
Talbot GH, Thye D, Das A, et al. Phase 2 study of ceftaro-line versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2007 Oct; 51(10): 3612–6
van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, et al. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci 2008 Mar; 29(3): 124–34
Zhanel GG, Karlowsky JA, Rubinstein E, et al. Tigecycline: a novel glycylcycline antibiotic. Expert Rev Anti Infect Ther 2006 Feb; 4(1): 9–25
Poulakou G, Giamarellou H. Investigational treatments for postoperative surgical site infections. Expert Opin Investig Drugs 2007 Feb; 16(2): 137–55
Corey R, Wilcox M, Talbot GH, et al. CANVAS-1: randomized, double-blinded, phase 3 study (P903-06) of the efficacy and safety of ceftaroline vs vancomycin plus aztreonam in complicated skin and skin structure infections (cSSSI) [abstract no. L-1515a]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Patrick G. An introduction to medicinal chemistry. 3rd ed. Oxford: Oxford University Press, 2005
Parish D, Scheinfeld N. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr Opin Investig Drugs 2008 Feb; 9(2): 201–9
Ishikawa T, Matsunaga N, Tawada H, et al. TAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physicochemical and pharmacological properties. Bioorg Med Chem 2003 May; 11(11): 2427–37
Page MGP. Emerging cephalosporins. Expert Opin Emerg Drugs 2007 Nov; 12(4): 511–24
Ikeda Y, Ban J, Ishikawa T, et al. Stability and stabilization studies of TAK-599 (ceftaroline fosamil), a novel N-phosphono type prodrug of anti-methicillin resistant Staphylococcus aureus cephalosporin T-91825. Chem Pharm Bull (Tokyo) 2008 Oct; 56(10): 1406–11
Villegas-Estrada A, Lee M, Hesek D, et al. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA β-lactam antibiotics. J Am Chem Soc 2008 Jul; 130(29): 9212–3
Moisan H, Pruneau M, Malouin F. Binding of ceftaroline (CPT) to penicillin-binding proteins (PBPs) of Streptococcus pneumoniae (SPN) and methicillin-resistant Staphylococcus aureus (MRSA) [abstract no. C1-183]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Hesek D, Suvorov M, Morio K, et al. Synthetic peptidoglycan substrates for penicillin-binding protein 5 of Gram-negative bacteria. J Org Chem 2004 Feb; 69(3): 778–84
Mushtaq S, Warner M, Ge Y, et al. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J Antimicrob Chemother 2007 Aug; 60(2): 300–11
Hinshaw RR, Schaadt RD, Murray B, et al. Spontaneous mutation frequency and serial passage resistance development studies with ceftaroline (CPT) [abstract no. C1-185]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Brown SD, Traczewski MM. Comparative in vitro antimicrobial activity of a new cephalosporin, ceftaroline, and determination of quality control ranges for MIC testing. Antimicrob Agents Chemother 2009 Mar; 53(3): 1271–4
Ge Y, Blosser RS, Sahm D, et al. In vitro activity of T-91825, a new anti-MRSA cephalosporin, against Gram-positive and Gram-negative clinical isolates. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2003 Sep 14–17; Chicago (IL)
Ge Y, Thye DA, Talbot GH. In vitro activity of ceftaroline against isolates from patients with complicated skin and skin structure infections (cSSSI) [abstract no. C2-864]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Ge Y, Biek D, Talbot G, et al. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob Agents Chemother 2008 Sep; 52(9): 3398–407
Iizawa Y, Nagai J, Ishikawa T, et al. In vitro antimicrobial activity of T-91825, a novel anti-MRSA cephalosporin, and in vivo anti-MRSA activity of its prodrug, TAK-599. J Infect Chemother 2004 Jun; 10(3): 146–56
Kaniga K, Redman R, Pecoraro ML, et al. Antibacterial activity of PPI-0903 against isolates from patients with complicated urinary tract infections (cUTI), complicated intra-abdominal infections (cIAI), and hospital-acquired pneumonia (HAP) [abstract no. F-1159]. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec 16–19; Washington, DC
McGee L, Biek D, Ge Y, et al. In vitro evaluation of the antimicrobial activity of ceftaroline against cephalosporin-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemotherapy 2009; 53: 552–6
Morrissey I, Curry J, Ge Y, et al. The activity of ceftaroline against community-acquired pneumonia (CAP) bloodstream isolates [abstract no. E-281]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Sader HS, Moet GJ, Fritsche TR, et al. Evaluation of the bactericidal activity of the novel cephalosporin ceftaroline (PPI-0903M) compared to ceftriaxone against Streptococcus pneumoniae [abstract no. E-121]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Sader HS, Fritsche TR, Jones RN. Antimicrobial activity of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2008 Mar; 52(3): 1153–5
Sader HS, Fritsche TR, Kaniga K, et al. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob Agents Chemother 2005 Aug; 49(8): 3501–12
Jones RN, Fritsche TR, Sader HS. Ceftaroline activity tested against organisms causing skin and skin structure infections (SSSI) isolated in USA and European medical centers in 2008 [abstract no. C1-160]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Sader HS, Fritsche TR, Jones RN. Antimicrobial activity of ceftaroline (CPT) tested against contemporary (2008) bacteria isolated from community-acquired respiratory tract infections, including oxacillin-(methicillin-) resistant Staphylococcus aureus (MRSA) [abstract no. C2-1974]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Patel SN, McGeer A, Green K, et al. Activities of ceftaroline, ceftobiprole, and cethromycin against multidrug resistant (MDR) Streptococcus pneumoniae isolates from Canadian Bacterial Surveillance Network (CBSN) [abstract no. C1-3843]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Barry PM, Lenderman CJ, Melendez JH, et al. In vitro activity of ceftaroline (CPT) against recent US isolates of Neisseria gonorrhoeae (NG) [abstract no. C1-163]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Vidaillac C, Leonard SN, Rybak MJ. In vitro activity of ceftaroline (CPT) vs vancomycin (VM) against MRSA and hVISA strains in a pharmacokinetic/pharmacodynamic (PK/PD) model [abstract no. A-979]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Saravolatz LD, Pawlak J, Johnson L. In vitro activity of ceftaroline against CA-MRSA, VISA, VRSA, and daptomycin-non-susceptible Staphylococcus aureus (DNSSA) [abstract no. C1-162]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Fenoll A, Aguilar L, Robledo O, et al. In vitro activity of ceftaroline against Streptococcus pneumoniae isolates exhibiting resistance to penicillin, amoxicillin, and cefotaxime. Antimicrob Agents Chemother 2008; 52: 4209–10
Jones RN, Fritsche TR, Ge Y, et al. Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity, effects of modifying in vitro testing parameters and optimization of disc diffusion tests. J Antimicrob Chemother 2005 Dec; 56(6): 1047–52
Sader HS, Moet G, Fritsche TR, et al. Evaluation of the bactericidal activity of the novel cephalosporin ceftaroline (PPI-0903M) compared to ceftriaxone against Streptococcus pneumoniae [abstract no. E-0121]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sept 27–30; San Francisco (CA)
Citron DM, Goldstein EJC. Effects of in vitro test method variables on ceftaroline activity against aerobic Gram-positive and Gram-negative pathogens [abstract no. D-2232]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC
Schaadt R, Sweeney D, Biek D, et al. The in vitro activity of ceftaroline in combination with other antibacterial agents [abstract no. E-279]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Vidaillac C, Leonard SN, Sader HS, et al. In vitro activity of ceftaroline (CPT) in combination against extended-spectrum β-lactamase (ESBL) producing Gram-negative bacteria (GN) [abstract no. C1-161]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Vidaillac C, Leonard SN, Rybak MJ. In vitro activity and aminoglycoside synergy of ceftaroline (CPT) against clinical isolates of hospital-acquired (HA) methicillin-resistant Staphylococcus aureus (MRSA) [abstract no. C1-3719]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting 2008 Oct 25–28; Washington, DC
Ge Y, Floren L, Redman R, et al. Single-dose pharmacokinetics (PK) of ceftaroline (PPI-0903) in healthy subjects [abstract no. A-1936]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Ge Y, Redman R, Floren L, et al. The pharmacokinetics (PK) and safety of ceftaroline (PPI-0903) in healthy subjects receiving multiple-dose intravenous (IV) infusions [abstract no. A-1937]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Riccobene T, Fang E, Thye D. A single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics (PK) of ceftaroline (CPT) administered by intramuscular (IM) injection to healthy subjects [abstract no. A-1888]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC
Ge Y, Thye D, Liao S, et al. Pharmacokinetics (PK) of ceftaroline (PPI0903) in subjects with mild or moderate renal impairment (RI) [abstract no. A-1939]. 46th Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Ge Y, Liao S, Talbot GH. Population pharmacokinetics (PK) analysis of ceftaroline (CPT) in volunteers and patients with complicated skin and skin structure infection (cSSSI) [abstract no. A-34]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Ge Y, Hubbel A. In vitro evaluation of plasma protein binding and metabolic stability of ceftaroline (PPI-0903) [abstract no. A-1935]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Jacqueline C, Caillon J, Miegeville A, et al. Penetration of ceftaroline (PPI-0903), a new cephalosporin, into lung tissues: measurement of plasma and lung tissue concentrations after a short IV infusion in the rabbit [abstract no. A-1938]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Ge Y, Liao S, Thye DA, et al. Ceftaroline (CPT) Dose adjustment recommendations for subjects with mild or moderate renal impairment (RI) [abstract no. A-35]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17-20; Chicago (IL)
Andes D, Craig WA. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 2006 Apr; 50(4): 1376–83
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–10
Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol 2004; 2: 289–300
Jacqueline C, Caillon J, Le Mabecque V, et al. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob Agents Chemother 2007 Sep; 51(9): 3397–400
Jacqueline C, Caillon J, Amador G, et al. In vivo assessment of the activity of ceftaroline, linezolid, and vancomycin in a rabbit osteomyelitis experimental model due to MRSA and GISA [poster no. 128-E]. American College of Clinical Pharmacy; 2008 Oct 19–22; Louisville (KY)
Xiong YQ, Li Y, Abdelsayed GA, et al. Real-time evaluation of ceftaroline (CPT), a new cephalosporin, vs vancomycin (VAN), and daptomycin (DAP) in a rat Staphylococcus aureus endocarditis model using in vivo bioluminescent imaging [abstract no. B-819]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Jacqueline C, Caillon J, LeMabecque V, et al. Evaluation of the efficacy of intramuscular (IM) administration of ceftaroline (CPT) against a methicillin-resistant Staphylococcus aureus (MRSA) strain in a rabbit endocarditis model (REM) [abstract no. B-1003]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Jacqueline C, Amador G, Batard E, et al. Assessment of the in vivo activity of ceftaroline (CPT) against vancomycin-susceptible and -resistant Enterococcus faecalis (EF) strains in a rabbit endocarditis model (REM): comparison with linezolid (LZO) and vancomycin (VAN) [abstract no. B-068]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Forest Laboratories Inc. Forest Laboratories announces positive results from phase III clinical studies of ceftaroline for the treatment of complicated skin and skin structure infections. Press release 2008 Jun 19 [online]. Available from URL: http://www.frx.com/news/PressRelease.aspx?ID=1167784 [Accessed 2008 Jul 11]
Levasseur P, Girard AM, Delachaume C, et al. NXL104, a novel β-lactamase inhibitor, restores the bactericidal activity of ceftazidime against ESBL and AmpC producing strains of Enterobacteriaceae [abstract no. F-127]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 27–30; San Francisco (CA)
Miossec C, Poirel L, Livermore D, et al. In vitro activity of the new β-lactamase inhibitor NXL104: restoration of ceftazidime (CAZ) efficacy against carbapenem-resistant Enterobacteriaceae strains [abstract no. F1-318]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Mushtaq S, Warner M, Miossec C, et al. NXL104/cephalosporin combinations vs. Enterobacteriaceae with CTX-M ESBLs [abstract no. F1-319]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17–20; Chicago (IL)
Novexel and Forest Laboratories Inc. Novexel and Forest Laboratories announce license agreement for NXL 104, a novel broad-spectrum beta-lactamase inhibitor. Press release 2008 Jan 22 [online]. Available from URL: http://www.reuters.com/article/pressRelease/idUS133612+22-Jan-2008+PRN20080122 [Accessed 2008 Jul 11]
Novexel. Novexel’s NXL104/ceftazidime combination commences phase II clinical trial in hospital patients with complicated urinary tract infections. Press release 2008 Nov 12 [online]. Available from URL: http://www.novexel.com/includes/cms/_contenus/mod_press_releases/CP_1211_2008.pdf. [Accessed 2009 Feb 23]
Acknowledgements
The authors would like to thank Mary Tarka for secretarial assistance. Editorial assistance was provided by Scientific Therapeutics Information, Inc., Springfield, New Jersey, USA, and was funded by Forest Laboratories, Inc., New York, USA. No financial support was received for authorship of this article. The authors have declared that they have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhanel, G.G., Sniezek, G., Schweizer, F. et al. Ceftaroline. Drugs 69, 809–831 (2009). https://doi.org/10.2165/00003495-200969070-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969070-00003