Abstract
Background and objective
A novel oral, extended-release, microsphere formulation of azithromycin (AZSR) was developed to improve the gastrointestinal tolerability profile while allowing administration of an entire treatment course of azithromycin in a single dose. Several phase I clinical pharmacology studies were conducted to (i) identify a well-tolerated single-dose formulation that met a predefined exposure target; and (ii) evaluate the effect of food and antacid on the absorption of this formulation. Of these, five pivotal studies are described here.
Methods
The pharmacokinetic profile of AZSR was compared with that of the commercially available immediate-release azithromycin formulation (AZM) in an open-label, crossover, single-dose study (Study A), and their gastrointestinal tolerability profiles were compared in an observer-blind, parallel group, single-dose study (Study B). The effects of food (a high-fat meal and a standard meal) and antacid (a single 20mL dose of Maalox® Regular Strength, containing magnesium hydroxide, aluminium hydroxide and simethicone) on the absorption of azithromycin from AZSR were evaluated in three separate open-label, crossover, single-dose studies (Studies C, D and E). Healthy adult subjects were enrolled in all five studies, and all subjects were evaluable for tolerability. The dose used for all azithromycin formulations was 2.0g. Serum azithromycin concentrations were determined using a validated high-performance liquid chromatography/electrochemical detection method, and pharmacokinetic parameters were analysed using noncompartmental methods.
Results
377 subjects received a single 2.0g dose of azithromycin as AZSR and/or AZM in the five studies. Compared with AZM, AZSR had a slower absorption rate (57% decrease in the mean peak concentration [Cmax] and an approximate 2.5-hour delay in the time to reach Cmax [tmax]), with a mean relative bioavailability of 82.8%, which met the predefined exposure target (at least 80% bioavailability relative to AZM). Compared with AZM, AZSR was associated with significantly lower rates of nausea and vomiting. A high-fat meal increased the mean area under the serum concentration-time curve [AUC] from time zero to 72 hours post-dose (AUC72h) by 23% and increased the Cmax of azithromycin by 115%. A standard meal increased the mean Cmax by 119% but had no clinically significant effect on the AUC72h. AZSR appeared to be better tolerated in the fasted state than in the fed state. The AUC72h and Cmax of AZSR were not significantly affected by co-administration with a single dose of antacid.
Conclusions
The extended-release microsphere formulation of azithromycin, AZSR, allows administration of an entire therapeutic course of azithromycin as a well-tolerated single 2.0g dose. This formulation should be administered on an empty stomach and can be co-administered with antacids.
Similar content being viewed by others
Background
Azithromycin is an azalide, structurally related to the macrolide family of antimicrobials, which acts by binding to the 50S ribosomal subunit of susceptible micro-organisms, thereby interfering with protein synthesis. It is approved worldwide for the treatment of a variety of community-acquired infections. Azithromycin is rapidly absorbed following oral administration and achieves sustained high concentrations in a variety of body tissues/fluids.[1] This property, coupled with a long serum terminal elimination half-life of approximately 68 hours and drug delivery to the site of infection through white blood cell trafficking, allows the use of short courses of therapy ranging from 1 to 5 days, depending on the underlying infection.[1–3] For most adult indications, a total of 1.5g of immediate-release (IR) azithromycin (AZM; Zithromax®)Footnote 1 is administered in divided doses over a period of either 3 days (500mg daily) or 5 days (500mg on day 1 and 250mg daily on days 2–5).[1]
Preclinical results in several animal infection models (including murine pneumonia, acute peritonitis and neutropenic thigh infection, and Haemophilus influenzae otitis media in gerbils) demonstrated that administration of the entire therapeutic course of azithromycin as a single dose appeared to produce superior rates of survival and more rapid bacterial clearance than the same total dose divided over multiple days.[2–5] These results suggest that ‘front-loading’, which achieves higher systemic exposure early during treatment (i.e. the area under the serum concentration-time curve [AUC] from time zero to 24 hours [AUC24] post-dosing), could result in more efficient bacterial killing. The correlation between azithromycin serum exposure and efficacy is not ideal since the azithromycin exposure at the tissue sites would be a better surrogate marker based on its unique pharmacokinetic characteristics. However, because of technical challenges in measuring exposure at the tissue sites, serum exposure is used instead for pharmacokinetic and pharmacodynamic correlation in most cases. Among global pharmacokinetic-pharmacodynamic indices, the ratio of the serum AUC to the minimum inhibitory concentration (MIC) has been reported to be the most predictive index of azithromycin efficacy in animal models.[2,5,6] Therefore, if a well-tolerated single-dose formulation could deliver an entire therapeutic course of azithromycin (i.e. the total systemic exposure of the established multiple-dose regimens), it would be expected to be efficacious. It should also achieve improved patient adherence.
The most common adverse events (AEs) in adult patients receiving multiple-dose regimens of AZM are related to the gastrointestinal tract, with diarrhoea/loose stools (5%), nausea (3%) and abdominal pain (3%) being most frequently reported.[1] The gastrointestinal AEs of AZM are dose-related. Previous studies with intravenous azithromycin have shown that the incidence of gastrointestinal AEs in patients receiving an intravenous regimen (500 mg/day) is no higher than that with oral regimens, although the intravenous dose produced several-fold higher serum azithromycin concentrations than the oral regimen (i.e. peak serum concentration [Cmax] 3.63 µg/mL vs 0.41 µg/mL).[1,7] This has suggested that the gastrointestinal AEs, such as nausea and vomiting, are primarily local in origin and occur shortly after oral dosing of azithromycin, possibly due to the drug’s action on the motilin receptors in the upper gastrointestinal tract, like other macrolides (i.e. erythromycin).[8,9] The highest single oral dose of AZM currently approved is 2.0g as a sachet of oral powder for suspension for the treatment of gonococcal urethritis and cervicitis. However, the actual use of this dose has been limited because of the high incidence of gastrointestinal AEs such as nausea (18%), diarrhoea/loose stools (14%) and vomiting (7%).[1]
An extended-release (ER) microsphere formulation of azithromycin (AZSR) was designed to deliver an entire therapeutic course in a single dose with a tolerability profile comparable to those of the multiple-dose AZM regimens. Two formulation strategies were pursued, which formed the basis for the ER formulation development program: (i) delaying the release of azithromycin so that it is released in the lower gastrointestinal tract and hence bypasses the upper gastrointestinal motilin receptors; and (ii) decreasing the release rate of azithromycin from the matrix so that a lesser amount of drug comes into contact with the upper gastrointestinal mucosa per unit time to reduce concentration-dependent local irritation. A microsphere formulation was chosen as the drug delivery platform since it could provide a convenient method for administration of a large dose as oral powder for suspension, control the drug release rate and mask the taste of azithromycin. In addition, to avoid substantial compromise of the bioavailability of azithromycin in the new formulation, a minimum systemic exposure target was predefined as achievement of at least 80% bioavailability relative to the IR formulation, with a lower limit of the 90% confidence interval (CI) of >70%. Several phase I clinical pharmacology studies were designed to: (i) identify an ER microsphere formulation (AZSR) that maintains at least 80% systemic exposure relative to AZM; (ii) prove the concept that controlled release of azithromycin in the gastrointestinal tract is associated with a lower incidence of gastrointestinal AEs; (iii) estimate the effect of food on the absorption of AZSR; and (iv) estimate the effect of antacid on the absorption of AZSR. Five pivotal studies are described here, including two formulation development studies (pharmacokinetic evaluation [Study A] and tolerability evaluation [Study B]), two food-effect studies (Studies C and D) and one antacid interaction study (Study E).
Methods
Subjects
All five studies had similar subject populations. Healthy male and female adult subjects of any race (aged 18–65 years and bodyweight ≥50kg) who were willing and able to provide written informed consent and to be confined to the Clinical Research Unit, as required by the study protocols, were included. Subjects were required to be in good health as determined by a detailed medical history, full physical examination including vital signs, 12-lead ECGs and clinical laboratory tests (including complete blood count, differential cell count, liver and renal function tests, and urinalysis). Subjects were excluded if they had any conditions possibly affecting drug absorption, had used prescription or non-prescription drugs or dietary supplements within 7 days prior to the first dose of study medication (excluding oral contraceptives, hormone replacement therapy and paracetamol [acetaminophen] ≤2 g/day), had used herbal supplements within 30 days, had a known allergy to macrolide antimicrobials or a severe allergic reaction to any drug in the past, or had a history of intolerance of azithromycin.
Treatments
AZSR was the primary ER microsphere formulation of azithromycin under investigation. The microspheres had a mean diameter ranging from approximately 100µm to 300µm, which was selected based on the combination of the microsphere performance (mouth feel of the suspension), release profile, and manufacturability. In addition, since the melting point of azithromycin dihydrate is 116°C, it is desirable that the carrier has a melting point of <113°C to ensure that azithromycin does not melt during the melt-congealing process. AZM, the IR oral powder for suspension formulation of azithromycin, was the reference standard. The dose used for all azithromycin formulations was 2.0g. The 20mL of antacid was supplied by the Clinical Research Unit as Maalox® Regular Strength cooling mint suspension containing magnesium hydroxide 800mg, aluminium hydroxide 800mg and simethicone 80mg (Novartis Consumer, Parsippany, NJ, USA). The US FDA-recommended high-fat meal (containing approximately 150 kcal of protein, 250 kcal of carbohydrate and 500–600 kcal of fat) and the standard meal (containing approximately 56 kcal of protein, 316 kcal of carbohydrate and 207 kcal of fat) were used in the food-effect studies.
Besides AZSR, another experimental microsphere formulation was also evaluated in Studies A and B. The performance of this experimental formulation is briefly discussed, in terms of its difference from AZSR, in the Discussion section.
Study Designs
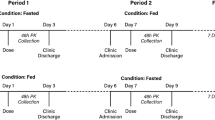
The study designs of these five studies are summarised in table I. Study A (pharmacokinetic evaluation) was a two-way, crossover, single-dose study with a washout period of at least 14–16 days between treatments. Serum samples were collected at time zero (just prior to dosing) and at 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 72 and 96 hours post-dose for azithromycin measurement in each dosing period. All subjects were evaluated for safety including clinical observation, limited physical examinations, and voluntary reporting of AEs. Study B (tolerability study) evaluated the study formulations in a parallel group design with extensive safety assessments in multiple study centres. Subjects were given the study drugs and kept in isolation from each other for at least 2 hours after dosing. The safety and tolerability of treatments were assessed during the study by clinical observation and by querying subjects for AEs at 1, 2, 4, 6, 8, 12 and 24 hours after dosing. Studies C, D and E (food-effect studies and an antacid interaction study) had a design similar to that of Study A, including crossover, pharmacokinetic sampling timepoints (except for the 96-hour timepoint) and safety assessments. With the exception of the fed cohorts in the food-effect studies, the subjects were administered the study drugs under fasted conditions. The subjects were fasted for at least 4 hours before any safety laboratory evaluations and for 8 hours prior to the start of drug administration, and were required to refrain from lying down, eating, and drinking beverages other than water during the first 4 hours after dosing. For all studies, the subjects were confined in the Clinical Research Unit for at least 24 hours following dosing. After discharge from the Clinical Research Unit on day 2, the subjects were instructed to notify the clinical sites of additional AEs for up to 35 days after the last dose of the study drug. All study protocols were approved by the local Institutional Review Board, including the Research Consultant’s Review Committee (Austin, TX, USA; Studies A, B, C and E), PRACS Institute Institutional Review Board (Fargo, ND, USA; Studies B and D), Independent Investigational Review Board (Plantation, FL, USA; Study B) and Quintiles Institutional Review Board (Lenexa, KS, USA; Study B). All five studies were conducted according to the Declaration of Helsinki and local laws and regulations relevant to the use of new therapeutic agents in the US.
Sample Size Determination
The objective of Study A was to estimate the oral bioavailability of a single 2.0g dose of AZSR relative to AZM, the commercial sachet, in a crossover design. With a sample size of 16 subjects (eight per sequence), if the relative bioavailability estimate was 80%, the 90% CI would be no wider than 70.2%, 91.2% with a tolerance probability of 0.80. This calculation was based on the assumption that the intrasubject coefficient of variance for the AUC was 32%, which was estimated from an experimental ER formulation in a pilot study.[10]
In Study B, it was determined that 100 subjects per group would be required to provide ≥80% power for a two-sided (α = 0.05) test to detect a difference in the following hypothesised rates of gastrointestinal AEs: 67% for AZM 2.0g vs 45% for AZSR 2.0g in a parallel group design. These hypothesised rates were based on preliminary results with AZM 2.0g in a pilot study.[10]
The objective of Study C was to estimate the oral bioavailability of a single 2.0g dose of AZSR when administered with a high-fat meal. With a sample size of 16 subjects and a tolerance probability of 0.80, if the relative bioavailability (fed/fasted) estimate was 80%, the 90% CI would be no wider than 67.2%, 95.2%; if the Cmax ratio estimate was 0.6, the 90% CI would be no wider than 46.2%, 78.0%. This calculation was based on the assumption that the intrasubject coefficients of variance for the AUC and Cmax were 25% and 38%, respectively, which was estimated from Study A.
In Study D, the sample size was computed using logarithmic transformation, t-statistics, a two-sided 90% CI, and 90% power to detect a food effect, with a bioequivalent acceptance interval (80%, 125%) in accordance with the FDA draft guidance.[11] The assumed intrasubject coefficient of variance for exposure parameters was 38% as a conservative estimate (data on Cmax from Study A). The ratio of the mean parameters (fed/fasted) was assumed to be 1.05. A sample size of 84 subjects (42 per sequence) or more provided the desired power. Eight additional subjects were enrolled to allow for discontinuations, giving a total of 92 enrolled subjects.
In Study E, the sample size was computed similarly to Study D: logarithmic transformation, t-statistics, a two-sided 90% CI, and at least 80% power to detect bioequivalence with an acceptance interval of 80%, 125% for the AUC, the primary endpoint. The assumed intrasubject coefficient of variance for the AUC was 30%, and it was assumed that the true mean AUC of AZSR with antacid differed from that of AZSR alone by ≤5%. A sample size of 40 subjects (20 per sequence) or more provided the desired power.
Drug Administration
Single 2.0g doses of AZSR and AZM were supplied as oral powder for suspension and were reconstituted with prescribed quantities of water (60mL) just prior to dosing. All azithromycin treatments were administered with water 240mL. In Study E with antacid, AZSR was administered immediately after antacid 20mL, followed by water 240mL.
Pharmacokinetic Assessments
Blood samples collected for azithromycin measurement were kept at room temperature for approximately 30 minutes until clotted and were centrifuged at approximately 1700 × g for 10 minutes at 4°C. Within 1 hour of collection, the serum was harvested and stored under refrigeration (at least −20°C) until analysed.
BAS Analytics (West Lafayette, Indiana, USA) analysed the serum samples for azithromycin using a validated high-performance liquid chromatography/electrochemical detection (LCEC) method similar to a previously reported method.[12] The N-propargyl derivative of azithromycin (CP-67094) was used as the internal standard. After the addition of 0.06 mol/L sodium carbonate 1.0mL solution and internal standard 50µL in 50% acetonitrile (v/v) to the serum sample (1.0mL), azithromycin was extracted into methyl t-butyl ether 2.0mL using a liquid-liquid extraction procedure. The upper ether layer was collected, evaporated under nitrogen in a 40–50°C water bath and reconstituted in 250µL of phosphate buffer/acetonitrile mixture (pH 6.0) [75: 25, v/v]. In order to eliminate late-eluting peaks in the chromatogram, the reconstituted extract was washed with hexane 500µL. A 60µL aliquot of the extract was injected into an LCEC system (a Bioanalytical Systems PM-80 isocratic pump with an LC-26A on-line vacuum degasser and a Bioanalytical Systems LC-4C electrochemical detector) set up with a ZirChrom PBD column (150 × 4.6mm, 3µm) and an alkaline phosphate buffer/acetonitrile (65: 35, v/v) mobile phase. Azithromycin and the internal standard were detected by electrochemical oxidation of their tertiary amine moiety on a glassy carbon electrode at 800mV with silver/silver chloride as the reference electrode. The recovery with the extraction procedure was 71.6% for azithromycin and 81.1% for the internal standard. The dynamic range of the serum assay was 0.0104–1.00 µg/mL. The accuracy of the quality control samples used during the sample analysis ranged from −7.7% to 4.2% with a precision of ≤7.0%. All samples were analysed within an established long-term stability period (95 days at −20°C and 4.5 years at −80°C).
Pharmacokinetic analyses were carried out using WinNonlin® Version 3.2 software (Pharsight Corporation, Mountain View, CA, USA) using the standard noncompartmental method. The primary azithromycin pharmacokinetic endpoints were the peak serum concentration (Cmax), the time to reach Cmax (tmax), and the AUC from time zero to the time of the last quantifiable concentration (AUClast). The Cmax and tmax were estimated directly from the observed concentration-time data. The AUClast was estimated using linear/log trapezoidal approximation. Since all subjects had measurable serum concentrations at the last sampling timepoint, the AUClast was equivalent to the AUC from time zero to 72 hours (AUC72h) or the AUC from time zero to 96 hours (AUC96h). The truncated AUC72h was used as the primary endpoint in studies C, D and E for comparison according to the FDA draft guidance on bioequivalence studies for orally administered drug products with a long elimination half-life.[11] Since Study A was the first study of an AZSR formulation in humans, the AUC96h was selected to assure the completion of gastrointestinal transit.
The log-transformed AUClast and Cmax were analysed using a mixed effects ANOVA model with a SAS Proc Mixed procedure (SAS Institute Inc., Cary, NC, USA). The sequence, period and treatment were considered fixed effects, and subjects within the sequence were considered a random effect. The point estimates of the adjusted mean differences (test-reference) and corresponding 90% CIs were estimated from these models with a restricted maximum likelihood (REML) estimation method, variance-covariance structure of compound symmetry, and the Satterthwaite adjustment for degrees of freedom. The differences and 90% CIs for the differences were anti-log transformed to derive estimates of the ratios of the adjusted geometric means (test/reference) and the corresponding 90% CIs for these ratios. In Studies C, D and E, the absence of a food effect or the lack of drug interaction was established if the 90% CIs of the geometric ratios for the AUC72h and Cmax were contained in the acceptance interval (80%, 125%).
In study A, the untransformed (raw) tmax was used and the 90% CI of the mean difference (AZSR - AZM) of the mean tmax was calculated. The lower limit of the 90% CI was used to determine whether and by how long the tmax was delayed with AZSR. No formal analysis of the tmax was conducted for the other studies.
Safety and Tolerability Assessments
Safety data were summarised descriptively and tabulated for each treatment for all studies, especially gastrointestinal AEs. Additionally, in Study B (the tolerability study), the difference between the proportion of subjects who received AZSR and experienced one or more gastrointestinal AEs (nausea, vomiting, diarrhoea and abdominal pain) and the proportion of subjects who received AZM and experienced the same gastrointestinal AEs was estimated. Inferential statistics and 95% CIs of the differences of these proportions were calculated (AZSR - AZM) using normal approximation to the binomial distribution with continuity correction, and the most severe data for each event were used in the analysis. For these AEs, adjustments for multiple comparisons were made using Bonferroni’s procedure. Using this adjustment, each comparison was considered to be statistically significant if the observed p-value was ≤0.025. Since the study was stratified by gender, a separate Cochran-Mantel-Haenszel test with adjustment for gender was performed for each of these comparisons.
Results
Subjects
Details of the demographic characteristics of the subjects enrolled in the five studies are shown in table II. A total of 377 subjects were enrolled and all of them were included in the evaluation of safety and tolerability. Five subjects (one in Study C given the high-fat meal and four in Study D given the standard meal) were excluded from the pharmacokinetic analysis because they withdrew from the study before completion of the pharmacokinetic sample collection. None of these withdrawals were due to treatment-related AEs. Overall, the mean age ranged from 25 to 34 years and the mean weight ranged from 66 to 72kg. The majority of the subjects were Caucasian.
Optimum Formulation Selection
Pharmacokinetic Evaluation
Study A evaluated the pharmacokinetic profiles of single 2.0g doses of AZSR and AZM in a two-way crossover design. AZSR had a significantly lower Cmax (mean decrease of 57%) and a delayed tmax (∼2.5 hours later) compared with AZM, indicating a slower absorption rate (figure 1 and table III). The mean relative bioavailability of AZSR was 82.8%, with the lower limit of the 90% CI for the geometric mean ratio of the AUC96h being >70% (70.8%), thus meeting the predefined exposure target.
Gastrointestinal Tolerability Evaluation
Study B evaluated the tolerability profiles of single 2.0g doses of AZSR and AZM in parallel groups. Most AEs were gastrointestinal in nature, and the most common ones for AZSR in descending order of incidence were abdominal pain, diarrhoea, nausea and headache. For AZM, they were nausea, abdominal pain, diarrhoea and vomiting (table IV). One non-gastrointestinal AE common to AZSR and AZM was headache (12% and 6%, respectively). Most AEs associated with AZSR and AZM were mild in intensity (98% and 92%, respectively).
The proportion of subjects who had single or combined gastrointestinal events of nausea, vomiting and/or diarrhoea was lower for AZSR compared with AZM. The frequency of the most relevant gastrointestinal AEs (nausea and/or vomiting) was significantly decreased (p < 0.0001) with AZSR compared with AZM under Bonferroni’s adjustment (α = 0.025) [table IV]. Compared with AZM, AZSR also appeared to be associated with a lower incidence of diarrhoea (p = 0.04). However, this difference did not attain the pre-specified level of statistical significance.
Based on the results of these two studies, AZSR was selected for further development. Factors such as food intake and antacids, which may have a deleterious impact on the gastrointestinal absorption of AZSR, were evaluated in subsequent studies.
Effect of Food on Absorption of Azithromycin from the Extended-Release Microsphere Formulation (AZSR)
A High-Fat Meal
Study C evaluated the effect of a high-fat meal on the absorption of AZSR 2.0g in 16 subjects. Fifteen subjects completed the study and were included in the pharmacokinetic analysis. The high-fat meal significantly increased the rate and extent of the absorption of azithromycin from AZSR, as evidenced by a 115% increase in the mean Cmax and a 23% increase in the mean AUC72h. The tmax was also shortened (∼4 hours earlier) [figure 2 and table V]. The 90% CI for the Cmax and AUC72h ratios fell out of the 80–125% acceptance interval for bioequivalence, indicating a significant food effect.
Mean serum azithromycin concentration-time profiles following a single 2.0g dose of the extended-release microsphere formulation of azithromycin in healthy adult subjects: (a) after fasting or after eating a high-fat meal (n = 15); and (b) after fasting or after eating a standard meal (n = 88). The error bars represent the standard deviation.
A Standard Meal
Study D evaluated the effect of a standard meal on the absorption of AZSR 2.0g in 92 subjects. Eighty-eight subjects completed the study and were included in the pharmacokinetic analysis. The standard meal significantly increased the rate of absorption of azithromycin from AZSR, as indicated by a 119% increase in the mean Cmax and an earlier tmax (∼2 hours earlier) [table V]. The 90% CI for the Cmax ratio fell out of the 80–125% acceptance interval for bioequivalence, indicating a significant food effect on the absorption rate. The mean AUC72h was increased by 12%, and its 90% CI fell within the 80–125% acceptance interval for bioequivalence, suggesting that the standard meal had no significant effect on the extent of absorption (table V).
The descriptive summary of AEs reported in these two food-effect studies is presented in table IV. The gastrointestinal-related AEs (e.g. nausea, diarrhoea and vomiting) were less frequent in subjects receiving AZSR under fasted conditions than with food. In Study D, which had a relatively large sample size (n = 92), this difference was more apparent although no formal statistical analysis was performed.
Effect of Antacids on Absorption of Azithromycin from AZSR
The effect of a single dose of regular-strength antacid on the absorption of azithromycin from AZSR was evaluated in 39 subjects (Study E). The azithromycin exposure parameters were similar when AZSR was administered with or without antacid, and the statistical analysis of the AUC72h and Cmax demonstrated bioequivalence (table V). AZSR was well tolerated and had similar tolerability profiles when administered with or without antacid (table IV).
Discussion
The development of the single-dose azithromycin regimen was based on preclinical findings and the corresponding pharmacokinetic-pharmacodynamic relationships.[2–5] Previously, for the macrolide class, the duration for which plasma drug concentration remained above the MIC was generally considered to correlate best with clinical efficacy, as observed with erythromycin and clarithromycin.[13] Recent research has demonstrated that the AUC/MIC ratio also appeared to correlate with the clinical outcome of clarithromycin.[13,14] For the subclass members of the macrolide family, such as azithromycin and telithromycin, the AUC/MIC or Cmax/MIC have been reported to correlate most closely with clinical efficacy, where the AUC/MIC and Cmax/MIC are highly correlated.[5,13,15] The pharmacokinetic-pharmacodynamic index of the AUC/MIC indicates that the key determinant for clinical efficacy is the dose, not the dosing interval, which makes short-course therapy possible. This property, coupled with other pharmacokinetic characteristics such as extensive distribution into tissues and white blood cells to reserve the drug in the body, could have prolonged persistent effects without frequent dosing. This has been confirmed by clinical efficacy data on azithromycin, clarithromycin and telithromycin with short-course therapy (3- to 7-day regimens) for the treatment of community-acquired infections compared with traditional 10- to 14-day regimens.[16–18] Furthermore, with its unique pharmacokinetic properties, azithromycin has demonstrated clinical efficacy with a single-dose regimen.[1,19–21]
The ER microsphere formulation was designed to prolong the release profile of azithromycin by incorporating alkalinising agents (sodium phosphate tribasic and magnesium hydroxide) in the formulation. The high pH of the constituted suspension minimises drug release from microspheres in the mouth and stomach, which helps bypass most of the motilin receptors in the upper gastrointestinal tract. It has been reported that motilin receptors are expressed in all parts of the gastrointestinal tract, with greater expression in muscle than in mucosa,[22,23] and the motilin receptor density is maximal in the antrum and generally decreases in the aboral direction (the receptor density, expressed as fmol/mg of protein, was 26 in the gastric corpus, 77 in the antrum and 49 in the duodenum).[24]
It has been demonstrated that AZSR decreased the absorption rate of azithromycin compared with AZM. Minimal absorption was observed in the first hour after dosing of AZSR. As shown in figure 1, the major difference in the pharmacokinetic profiles was within the first 6 hours after dosing. The drug concentration-time profiles after 12 hours were similar. The lower bioavailability of AZSR relative to AZM is probably due to the slow release of azithromycin, which causes azithromycin to bypass a small portion of the absorption site(s) in the gastrointestinal tract. It is considered that azithromycin is mainly absorbed in the small intestine, although an earlier exploratory gastrointestinal intubation study in healthy subjects showed that dosing of azithromycin 2.0g directly into the ileocecal region of the intestine resulted in comparable systemic exposure but a lower incidence of nausea and vomiting than when azithromycin was administered directly into the duodenum.[25]
AZSR was also found to have a more favourable tolerability profile than AZM, especially in terms of being associated with a significantly lower incidence of nausea and vomiting (p < 0.0001). In addition, about 90% of all nausea and/or vomiting events in all five studies occurred within the first 2 hours after dosing. Hence, the delayed absorption of AZSR coupled with the improved gastrointestinal tolerability support the hypothesis that less contact between azithromycin and motilin receptors in the upper gastrointestinal tract leads to decreased incidence of nausea and vomiting.
Besides AZSR, several other experimental ER microsphere formulations were also evaluated in the development program. For instance, an experimental ER formulation tested in parallel with AZSR in Studies A and B had a composition similar to AZSR with the exception that it did not contain magnesium hydroxide. This experimental formulation also had a slower absorption rate of azithromycin, but its mean relative bioavailability was 72.8%, which was lower than that of AZSR. The tolerability profiles of AZSR and the experimental formulation were comparable. Since azithromycin is known to undergo cleavage of its cladinose sugar moiety to produce descladinose-azithromycin at acidic pH levels,[26] it was speculated that the additional magnesium hydroxide might protect azithromycin from acidic degradation in the gastrointestinal tract so that more intact azithromycin would be available for absorption, resulting in higher systemic exposure.
Based on the pharmacokinetic and gastrointestinal tolerability profiles, AZSR was selected as the formulation for phase III clinical studies and, subsequently, as the commercial formulation. The single 2.0g oral dose AZSR regimen (Zmax™) was approved by the FDA in June 2005 for the treatment of community-acquired pneumonia and acute bacterial sinusitis in adults.[21] The most commonly reported AEs for this regimen are diarrhoea/loose stools (11.6%), nausea (3.9%), abdominal pain (2.7%) and vomiting (1.1%).[21] Based on the data of well-controlled phase III studies, the safety and tolerability profile of AZSR 2.0g is comparable to the multiple-dose regimens of IR formulations, with the exception of a higher rate of diarrhoea/loose stools, which is likely due to high local gastrointestinal exposure to azithromycin.[1,21]
It should be noted that the incidences of gastrointestinal AEs for single 2.0g doses of AZSR and AZM reported by healthy subjects in the tolerability study were about 2- to 3-fold higher than those reported by the patient population in well-controlled phase III studies.[1,21] These higher AE incidences in healthy subjects in a phase I setting are not unexpected, as subjects are under continuous observation and are queried about AEs on a frequent basis. Notably, the differences in the gastrointestinal AE incidences reported for AZSR versus AZM in the tolerability study (table IV) were consistent with those reported in well-controlled phase III studies.[1,21] For instance, the incidence of nausea reported for AZSR and AZM in the tolerability study was 17% and 55%, respectively, and the corresponding incidence in the phase III studies was 4% and 18%, respectively. The incidence of vomiting in the tolerability study was 4% and 26%, respectively, and the corresponding incidence in the phase III studies was 1% and 7%, respectively.[1,21]
Across all five phase I studies, most AEs were gastrointestinal in nature, and the majority of events were graded as mild to moderate in severity. There were no deaths, treatment-related serious AEs or serious AEs resulting in discontinuation of treatment.
Both the high-fat meal and the standard meal had a more significant effect on the rate of absorption of azithromycin from AZSR (the Cmax being approximately 2-fold higher) than on the extent of absorption. Food intake diminished the ER characteristics of AZSR and was associated with an increase in the incidences of nausea and vomiting. This was most likely due to meal-triggered gastric acid secretions, which lead to faster release of azithromycin from the microspheres. Because of the decreased tolerability of AZSR when taken with food, it is recommended that AZSR be taken on an empty stomach.
Co-administration with 20mL of regular-strength antacid had no significant effect on the pharmacokinetic profile of AZSR even though antacids have an effect on the pH in the upper gastrointestinal tract.[27] It was suggested that the effect of antacids on the pH in the gastrointestinal tract is probably less significant than that of the alkalinising agents contained in the AZSR formulation and therefore antacid had minimal impact on the absorption of azithromycin from AZSR. Data on other alkalinising agents, such as histamine H2 receptor antagonists and proton pump inhibitors, are not available at present. The in vitro dissolution data showed that azithromycin release from AZSR is pH dependent, i.e. in 30 minutes 50–60% of azithromycin was released at pH 4.5–6.0 and <50% was released at pH 6.8.[28] Since the major absorption site for azithromycin is in the small intestine, it is speculated that the H2 receptor antagonists and proton pump inhibitors would not have a marked impact on the absorption of azithromycin from AZSR unless these agents significantly increased the pH in the small intestine.
A single 2.0g dose of AZSR results in a total azithromycin serum AUC comparable to that achieved with either the 3- or 5-day IR regimens (1.5g total dose).[21,29] Importantly, however, it provides a significant advantage over the multiple-dose IR regimens with respect to exposure within the first 24 hours in that the AUC24 and Cmax on the day of dosing are 2- to 3-fold higher.[21,29] A recently completed study directly compared the pharmacokinetics of the single 2.0g dose of AZSR and the 3-day IR tablet regimen (500mg once daily for 3 days) in both serum and white blood cells in healthy adult subjects.[30] The results demonstrated that a single 2.0g dose of AZSR achieved an approximately 3-fold higher azithromycin AUC24 in both serum and white blood cells, as well as an approximately 2-fold higher Cmax on day 1 compared with the 3-day tablet regimen. This ‘front-loading’ of the dose maximises drug exposure at a time when the bacterial burden is likely to be highest.
Conclusion
The extended-release microsphere formulation of azithromycin, AZSR, allows administration of an entire therapeutic course of azithromycin as a well-tolerated single 2.0g dose. This formulation should be administered on an empty stomach and can be co-administered with antacids.
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Pfizer, Inc. Zithromax® (azithromycin tablets) and (azithromycin for oral suspension) [online]. New York (NY): Pfizer, Inc., 2004. Available from URL: http://www.pfizer.com/pfizer/download/uspi_zithromax.pdf [Accessed 2007 Jan 30]
Drusano GL, Craig WA. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J Chemother 1997; 9 (3Suppl.): 38–44
Foulds G, Johnson RB. Selection of dose regimens of azithromycin. J Antimicrob Chemother 1993; 31 (ESuppl.): 39–50
Girard D, Bergeron JM, Milisen WB, et al. Comparison of azithromycin, roxithromycin, and cephalexin penetration kinetics in early and mature abscesses. J Antimicrob Chemother 1993; 31 (ESuppl.): 17–28
Girard D, Finegan SM, Dunne MW, et al. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J Antimicrob Chemother 2005; 56: 365–71
Craig WA. The hidden impact of antibacterial resistance in respiratory tract infection: re-evaluating current antibiotic therapy. Respir Med 2001; 95(A Suppl.): S12–9
Pfizer, Inc. Zithromax® (azithromycin for injection): for IV infusion only [online]. New York (NY): Pfizer, Inc., 2003. Available from URL: http://www.pfizer.com/pfizer/download/uspi_zithromaxIV.pdf [Accessed 2007 Jan 30]
Periti P, Mazzei T, Mini E, et al. Adverse effects of macrolide antibacterials. Drag Saf 1993; 9: 346–64
Weber Jr FH, Richards RD, McCallum RW. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent. Am J Gastroenterol 1993; 88: 485–90
Data on file, Pfizer Inc., 2002
US FDA. Draft guidance for industry: bioavailability and bio-equivalence studies for orally administered drag products — general considerations [online]. Rockville (MD): US FDA, 2002. Available from URL: http://www.fda.gov/cder/guidance/4964dft.htm [Accessed 2007 Jan 30]
Taninaka C, Ohtani H, Hanada E, et al. Determination of erythromycin, clarithromycin, roxithromycin, and azithromycin in plasma by high-performance liquid chromatography with amperometric detection. J Chromatogr B Biomed Sci Appl 2000; 738: 405–11
Nicolau DP. Predicting antibacterial response from pharmacodynamic and pharmacokinetic profiles. Infection 2001; 29 (2Suppl.): 11–5
Noreddin AM, Roberts D, Nichol K, et al. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob Agents Chemother 2002; 46: 4029–34
Zhanel GG, Johanson C, Hisanaga T, et al. Pharmacodynamic activity of telithromycin against macrolide-susceptible and macrolide-resistant Streptococcus pneumoniae simulating clinically achievable free serum and epithelial lining fluid concentrations. J Antimicrob Chemother 2004; 54: 1072–7
Darkes MJ, Perry CM. Clarithromycin extended-release tablet: a review of its use in the management of respiratory tract infections. Am J Respir Med 2003; 2: 175–201
Perez-Gorricho B, Ripoll M. Does short-course antibiotic therapy better meet patient expectations? Int J Antimicrob Agents 2003; 21: 222–8
Segreti J, House HR, Siegel RE. Principles of antibiotic treatment of community-acquired pneumonia in the outpatient setting. Am J Med 2005; 118(7A Suppl.): 21S–8S
Azithromycin extended-release (Zmax) for sinusitis and pneumonia. Med Lett Drags Ther 2005; 47: 78-80
Drehobl MA, De Salvo MC, Lewis DE, et al. Single-dose azithromycin microspheres vs clarithromycin extended release for the treatment of mild-to-moderate community-acquired pneumonia in adults. Chest 2005; 128: 2230–7
Pfizer, Inc. Zmax™ (azithromycin extended release) for oral suspension [online]. New York (NY): Pfizer, Inc., 2005. Available from URL: http://www.pfizer.com/pfizer/download/uspi_zmax.pdf [Accessed 2007 Jan 30]
Feighner SD, Tan CP, McKee KK, et al. Receptor for motilin identified in the human gastrointestinal system. Science 1999; 284: 2184–8
Takeshita E, Matsuura B, Dong M, et al. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol 2006; 41: 223–30
Peeters TL, Bormans V, Vantrappen G. Comparison of motilin binding to crude homogenates of human and canine gastrointestinal smooth muscle tissue. Regul Pept 1988; 23: 171–82
Data on file, Pfizer Inc., 1994
Fiese EF, Steffen SH. Comparison of the acid stability of azithromycin and erythromycin A. J Antimicrob Chemother 1990; 25 (ASuppl.): 39–47
Hurlimann S, Michel K, Inauen W, et al. Effect of Rennie Liquid versus Maalox Liquid on intragastric pH in a double-blind, randomized, placebo-controlled, triple cross-over study in healthy volunteers. Am J Gastroenterol 1996; 91: 1173–80
Data on file, Pfizer Inc., 2004
Amsden GW, Nafziger AN, Foulds G. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob Agents Chemother 1999; 43: 163–5
Liu P, Allaudeen H, Chandra R, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother 2007 Jan; 51(1): 103–9
Acknowledgements
We thank Dr T. Hunt and the staff of PPD Development, LLC, Dr A.K. Copa and the staff of PRACS Institute, Dr J. Carlson (Fargo, ND, USA), Dr M. Gutierrez (Fort Lauderdale, FL, USA), Dr A. Laurent (Austin, TX, USA) and Dr N. Abdou (Lenexa, KA, USA) and their staff for the clinical conduct of these studies. We thank our assay specialist, Penelope Crownover, and BAS Analytics (West Lafayette, IN, USA) for the analytical assay support. All of the authors are employees of Pfizer, Inc. and are eligible to receive Pfizer stock and stock options. All studies were sponsored by Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandra, R., Liu, P., Breen, J.D. et al. Clinical Pharmacokinetics and Gastrointestinal Tolerability of a Novel Extended-Release Microsphere Formulation of Azithromycin. Clin Pharmacokinet 46, 247–259 (2007). https://doi.org/10.2165/00003088-200746030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200746030-00005