Abstract
Objectives
The objectives of this research were to (1) assess the relative bioavailability following administration of a 100mg cilostazol suspension versus 100mg tablet; (2) assess dosage form equivalency (2 × 50mg compared with 1 × 100mg); (3) compare the relative bioavailability following a single 50mg dose of cilostazol administered as an ethanolic solution versus a 50mg tablet; and (4) determine the effects of high fat diet on the pharmacokinetics of cilostazol following a single dose of 100mg cilostazol in the fed or fasted state. Results were compiled from 3 separate studies to address these objectives.
Design
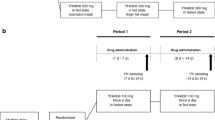
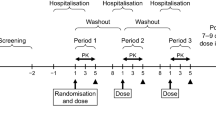
All studies involved healthy adult males receiving single oral doses of cilostazol in the fed or fasted state. The fed state consisted of administering cilostazol after ingestion of a high fat meal. One study compared the relative bioavailability of 100mg suspension and 2 × 50mg tablet versus 100mg tablet in a randomised crossover design. The study involving administration of a 50mg cilostazol ethanolic solution was a single treatment study. The effects of food on the pharmacokinetics of cilostazol after administration of 100mg cilostazol in the fed or fasted state as well as the pharmacokinetic profile following administration of a single 50mg oral dose of cilostazol were assessed in a randomised crossover design.
Study Participants
All participants were healthy nonsmoking males aged between 19 and 48 years whose bodyweight was within 15% of ideal bodyweight.
Main Outcome Measures
Noncompartmental pharmacokinetic parameters were determined for each study participant.
Results
The area under the plasma concentration-time curve (AUC) parameters were within the 80 to 125% criterion for bioequivalence for the cilostazol and its primary metabolite, OPC-13015. The maximum observed plasma concentrations (Cmax) for these formulations were not equivalent and indicated that the absorption of cilostazol from a suspension is more rapid than from a tablet. The apparent terminal half-lives (t1/2z) of cilostazol and OPC-13015 were shorter after administration of the suspension compared with the tablet. Cmax and AUC following administration of a single 50mg cilostazol tablet were approximately 80% of that from the same dose administered as an ethanolic solution. The t1/2z of cilostazol decreased from 15.5 hours after a tablet to 2.5 hours after an ethanolic solution. Upon coadministration with a high fat meal, the Cmax of cilostazol increased 90% and AUC∞ increased 25% (p < 0.05). The t1/2z decreased from 15.1 ± 14.5 hours (mean ± SD) in the fasted state to 5.4 ± 2.0 hours in the fed state. Single oral doses of 50 and 100mg cilostazol were well tolerated.
Conclusions
The relative bioavailability of the 100mg cilostazol tablet versus an oral 100mg cilostazol suspension is 100%.The 2 × 50mg and 1 × 100mg tablets are considered to be bioequivalent. The absorption following administration of 50mg cilostazol ethanolic solution is faster and appears to be greater than that after administration of the 50mg tablet. Coadministration of food increases the rate and extent of cilostazol absorption. The oral pharmacokinetics of cilostazol and metabolites are absorption-rate limited. The significant differences in the t1/2z observed when comparing cilostazol tablet, suspension, and solution as well as the effects of food suggest ‘flip-flop’ pharmacokinetics.
Similar content being viewed by others
References
Shimizu T, Osumi T, Niimi K, et al. Physicochemical properties and stability of cilostazol. Arzneimittelforschung 1985; 35(II): 1117–23.
Fu CJ, Tata PNV, Bramer SL. Simultaneous quantitative determination of cilostazol and its metabolites in plasma by high performance liquid chromatography. J Chromatogr B Biomed Sci App 1999; 728(2): 251–62.
Jusko WJ. Guidelines for collection and analysis of pharmacokinetic data. In: Evans WE, Jusko WJ, Schentag JJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 3rd ed. Vancouver: Applied Therapeutics, 1992; 2: 1–43.
Bramer SL, Forbes WP, Mallikaarjun S. Cilostazol pharmacokinetics after single and multiple oral doses in healthy males and patients with intermittent claudication resulting from peripheral arterial disease. Clin Pharmacokinet 1999; 37 Suppl. 2: 1–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bramer, S.L., Forbes, W.P. Relative Bioavailability and Effects of a High Fat Meal on Single Dose Cilostazol Pharmacokinetics. Clin Pharmacokinet 37 (Suppl 2), 13–23 (1999). https://doi.org/10.2165/00003088-199937002-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199937002-00002