Abstract
Antibacterials play a central role in the medical management of patients with cystic fibrosis (CF). Administration of adequate dosages of antibacterials results in pronounced beneficial effects on the morbidity and mortality of this patient group. The dosage of the antibacterial that is needed for optimal treatment depends on the individual patient’s pharmacokinetics and the pharmacokinetic-pharmacodynamic effect on the micro-organisms of relevance in the host.
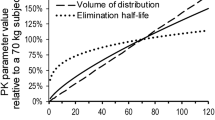
In general, the disposition of antibacterial drugs in patients with CF is not as ‘atypical’ as once thought. Recent research with adequately matched controls demonstrated that, for a few ß-lactam antibacterials only, a CF-specific increase of the total body clearance seems to exist and that the large volumes of distribution observed are the result of malnutrition and the relative lack of adipose tissue.
Pharmacokinetic-pharmacodynamic relationships in patients with CF are less well studied. Apart from the pharmacokinetics, there is a need for optimisation of antibacterial therapy. For the aminoglycosides, pharmacokinetic optimisation based on measured serum drug concentrations is common practice. The Sawchuk-Zaske method based on peak and trough drug concentrations is widely used.
A more sophisticated approach is the ‘goal-oriented model-based Bayesian adaptive control’ method, where integration of mathematically determined optimally (D-optimally) sampled serum drug concentrations and a population model results in the most likely set of individual pharmacokinetic parameter values suitable for further pharmacokinetic optimisation of the therapy. A future development is the integration of changing serum drug concentrations and killing rates of the target micro-organism to a pharmacokinetic-pharmacodynamic surrogate relationship to optimise drug therapy. The latter approach may be extremely useful in deciding on the frequency of aminoglycoside administration as well as the optimal use of the β-lactam antibacterials and fluoroquinolones.
Similar content being viewed by others
References
Boat TF, Welsh MJ, Beaudet AL. Cystic fibrosis. In: Scriver CL, Beaudet AL, Sly WS, et al., editors. The metabolic basis of inherited disease. Vol. 2. 6th ed. New York: McGraw-Hill, 1989: 2649–80.
Webb AK. The difficulties of treating infection in adults with pcystic fibrosis. Monaldi Arch Chest Dis 1993; 6: 657–61.
Mouton JW, den Hollander JG, Horrevorts AM. Emergence of resistance of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother 1993; 31: 919–26.
Høiby N. Antibiotic therapy for chronic infection of Pseudomonas in the lung. Annu Rev Med 1993; 44: 1–10.
Muhdi K, Edenborough FP, Gumery L, et al. Outcome for patients colonised with Burkholderia cepacia in a Birmingham adult cystic fibrosis clinic and the end of an epidemic. Thorax 1996; 51: 374–7.
Høiby N. Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multiresistant Pseudomonas aeruginosa. Neth J Med 1995; 46: 280–7.
Regelmann WE, Elliott GR, Warwick WJ, et al. Reduction of sputum pseudomonas aeruginosa density by antibiotics improve lung function in cystic fibrosis more than do broncho-dilators and chest physiotherapy alone. Am Rev Respir Dis 1990; 141: 913–21.
Webb AK, David TJ. Clinical management of children and adults with cystic fibrosis. BMJ 1994; 308: 459–62.
Mouton JW, Kerrebijn KF. Antibacterial therapy in cystic fibrosis. Med Clin North Am 1990; 74: 837–50.
Kuhn RJ, Nahata MC. Therapeutic management of cystic fibrosis. Clin Pharm 1985; 4: 555–65.
De Groot R, Smith AL. Antibiotic pharmacokinetics in cystic fibrosis: differences and clinical significance. Clin Pharmacokinet 1987; 13: 228–53.
Prandota J. Clinical pharmacology of antibiotics and other drugs in cystic fibrosis. Drugs 1988; 35: 542–78.
Horrevorts AM, Driessen O, Michel MF. Pharmacokinetics of antimicrobial drugs in cystic fibrosis. Chest 1988; 94 Suppl.: 120–5.
Spino M. Pharmacokinetics of drugs in cystic fibrosis. In: Gershwin E, editor. Clinical reviews in allergy. Vol. 9. Totowa (NJ): The Humana Press, 1991: 169–210.
Lindsay CA, Bosso JA. Optimisation of antibiotic therapy in cystic fibrosis patients: pharmacokinetic considerations. Clin Pharmacokinet 1993; 24: 496–506.
Touw DJ. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm World Sci 1998; 20: 149–60.
Levy J, Smith AL, Koup JR, et al. Disposition of tobramycin in patients with cystic fibrosis: a prospective controlled study. J Pediatr 1984; 105: 117–24.
Kearns GL, Hilman BC, Wilson JT. Dosing implications of altered gentamicin disposition in patients with cystic fibrosis. J Pediatrics 1982; 100: 312–8.
Vinks AATMM, Rossem RN, Mathot RAA, et al. Pharmacokinetics of aztreonam after single dose compared to matched controls and during home treatment by continuous infusion in patients with cystic fibrosis. In: Vinks AATMM, editor. Strategies for pharmacokinetic optimization of continuous infusion therapy of ceftazidime and aztreonam in patients with cystic fibrosis [thesis]. Leiden: University of Leiden, 1996.
MacDonald NE, Anas NG, Peterson RG, et al. Renal clearance of gentamicin in cystic fibrosis. J Pediatr 1983; 103: 985–90.
Spino M, Chai RP, Isles AF, et al. Assessment of glomerular filtration rate and effective renal plasma flow in cystic fibrosis. J Pediatr 1985; 107: 64–70.
Hedman A, Adan-Abdi Y, Alvan G, et al. Influence of the glomerular filtration rate on renal clearance in cystic fibrosis. Clin Pharmacokinet 1988; 15: 57–65.
Wang JP, Unadkat JD, Al-Habet SMH, et al. Disposition of drugs in cystic fibrosis. IV Mechanisms for enhanced renal clearance of ticarcillin. Clin Pharmacol Ther 1993; 54: 293–302.
Arvidsson A, Alvan G, Strandvik B. Difference in renal handling of cefsulodin between patients with cystic fibrosis and normal subjects. Acta Paediatr Scand 1983; 72: 293–4.
Bins JW, Mattie H. The tubular excretion of benzylpenicillin in patients with cystic fibrosis. Br J Clin Pharmacol 1989; 27: 291–4.
Hutabarat RM, Unadkat JD, Sahajwalla C, et al. Disposition of drugs in cystic fibrosis: I. Sulphamethoxazole and trimethoprim. Clin Pharmacol Ther 1991; 49: 402–9.
Reed MD, Stern RC, Myers C, et al. Lack of unique ciprofloxacin pharmacokinetic characteristics in patients with cystic fibrosis. J Clin Pharmacol 1988; 28: 691–9.
Mimeault J, Vallee F, Seelman R, et al. Altered disposition of fleroxacin in patients with cystic fibrosis. Clin Pharmacol Ther 1990; 47: 618–28.
Spino M, Chai RP, Isles AF, et al. Cloxacillin absorption and disposition in cystic fibrosis. J Pediatr 1984; 105: 829–35.
Demko CA, Thomassen MJ. Effect of mucoid property on antibiotic susceptibility of Pseudomonas aeruginosa. Curr Top Microbiol Immunol 1980; 4: 69–73.
Potter JL, Mathews LW, Spector S, et al. Complex formation between basic antibiotics and deoxyribonucleic acid in human pulmonary secretions. Pediatrics 1965; 36: 714–20.
Hunt BE, Weber A, Berger A, et al. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother 1995; 39: 34–9.
Reed MD, Stern RC, O’Brien CA, et al. Randomized doubleblind evaluation of ceftazidime dose ranging in hospitalized patients with cystic fibrosis. Antimicrob Agents Chemother 1987; 31: 698–702.
De Boeck K, Breysem L. Treatment of Pseudomonas aeruginosa lung infection in cystic fibrosis with high or conventional doses of ceftazidime. J Antimicrob Chemother 1998; 41: 407–9.
Touw DJ, Brimicombe RW, Hodson ME, et al. Inhalation of antibiotics in cystic fibrosis. Eur Respir J 1995; 8: 1594–604.
Kelly HW, Menendez R, Fan L, et al. Pharmacokinetics of tobramycin in cystic fibrosis. J Pediatr 1982; 100: 318–21.
Bauer LA, Piecoro JJ, Wilson HD, et al. Gentamicin and tobramycin pharmacokinetics in patients with cystic fibrosis. Clin Pharmacokinet 1983; 2: 262–4.
Hsu M-C, Aguila HA, Schmidt VL, et al. Individualization of tobramycin dosage in patients with cystic fibrosis. Pediatr Infect Dis 1984; 3: 526–9.
Horrevorts AM, Degener JE, Dzoljic-Danilovic G, et al. Pharmacokinetics of tobramycin in patients with cystic fibrosis: implications for the dosing interval. Chest 1985; 88: 260–4.
Michalsen H, Bergan T. Pharmacokinetics of netilmicin in children with and without cystic fibrosis. Antimicrob Agents Chemother 1981; 19: 1029–31.
Bosso JA, Townsend PL, Herbst JJ, et al. Pharmacokinetics and dosage requirements of netilmicin in cystic fibrosis patients. Antimicrob Agents Chemother 1985; 28: 829–31.
Mann HJ, Canafax DM, Cipolle RJ, et al. Increased dosage requirements of tobramycin and gentamicin for treating Pseudomonas pneumonia in patients with cystic fibrosis. Pediatr Pulmonol 1985; 1: 238–43.
Touw DJ, Vinks AATMM, Heijeman GBM, et al. Validation of tobramycin monitoring in adolescent and adult patients with cystic fibrosis. Ther Drag Monit 1993; 15: 52–9.
Touw DJ, Vinks AATMM, Heijeman HGM, et al. Suggestions for the optimization of the initial tobramycin dose in adolescent and adult patients with cystic fibrosis. Ther Drag Monit 1994; 16: 125–31.
Bates RD, Jones JW, McCoy K, et al. Pharmacokinetics and safety of tobramycin after once-daily administration in patients with cystic fibrosis [abstract. Pharmacotherapy 1995; 15: 392.
Touw DJ, Vinks AATMM, Heijeman HGM, et al. Prospective evaluation of a dose prediction algorithm for intravenous tobramycin in adolescent and adult patients with cystic fibrosis. Ther Drug Monit 1996; 18: 118–23.
Macdonald NE, Morris RF, Peterson RG. Disease severity as a factor in elimination of tobramycin (tobra) in patients with cystic fibrosis (CF) [abstract. Pediatr Res 1987; 21: 238A.
Horner GW, Stempel DA. Tobramycin elimination rate change from first to later doses in older cystic fibrosis patients. Drug Intell Clin Pharm 1987; 21: 276–8.
Autret E, Marchand S, Breteau M, et al. Pharmacokinetics of amikacin in cystic fibrosis: a study of bronchial diffusion. Eur J Clin Pharmacol 1986; 31: 79–83.
Vic P, Ategbo S, Turck D, et al. Tolerance, pharmacokinetics and efficacy of once daily amikacin for treatment of Pseudomonas aeruginosa pulmonary exacerbations in cystic fibrosis patients. Eur J Pediatr 1996; 155: 948–53.
Canis F, Husson MO, Turck D, et al. Pharmacokinetics and bronchial diffusion of single daily dose amikacin in cystic fibrosis patients. J Antimicrob Chemother 1997; 39: 431–3.
Vogelstein B, Kowarski A, Lietman PS. The pharmacokinetics of amikacin in children. J Pediatr 1977; 91: 333–9.
Beringer PM, Vinks AATMM, Jelliffe RW. Pharmacokinetics of once-daily amikacin dosing in patients with cystic fibrosis. J Antimicrob Chemother 1998; 41: 142–4.
Zach MS. Antibiotic treatment. Aerosol therapy [discussion]. Chest 1988; 94 Suppl.: 160S–1S.
Smith AL, Ramsey BW, Hedges DL, et al. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr Pulmonol 1989; 7: 265–71.
Mukhopadhyay S, Baer S, Blanshard J, et al. Assessment of potential ototoxicity following high-dose nebulized tobramycin in patients with cystic fibrosis. J Antimicrob Chemother 1993; 31: 429–36.
Cooney GF, Lum BL, Tomaselli M, et al. Absolute bioavailability and absorption characteristics of aerosolized tobramycin in adults with cystic fibrosis. J Clin Pharmacol 1994; 34: 255–9.
Weber A, Williams-Warren J, Ramsey B, et al. Tobramycin serum concentrations after aerosol and oral administration in cystic fibrosis. Am J Ther 1995; 2: 81–7.
Touw DJ, Jacobs FAH, Brimicombe RW, et al. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis. Antimicrob Agents Chemother 1997; 41: 184–7.
Touw DJ, De Graaf AL, De Goede PNFC. Evaluation of a fluorescence polarographic immunoassay with increased sensitivity for measurement of low concentrations of tobramycin in serum. Ther Drag Monit 1996; 18: 189–93.
Le Conte P, Potel G, Peltier P, et al. Lung distribution and pharmacokinetics of aerosolized tobramycin. Am Rev Respir Dis 1993; 147: 1279–82.
Jusko WJ, Mosovich LL, Gerbracht LM, et al. Enhanced renal excretion of dicloxacillin in patients with cystic fibrosis. Pediatrics 1975; 56: 1038–44.
Yaffe SJ, Gerbracht LM, Mosovich LL, et al. Pharmacokinetics of methicillin in patients with cystic fibrosis. J Infect Dis 1977; 135: 828–31.
Bergan T, Michalsen H. Pharmacokinetics of azlocillin in children with cystic fibrosis. Arzneimittel Forschung 1979; 29: 1955–7.
Martini N, Agostini M, Barlocco G, et al. Serum and sputum concentrations of azlocillin, cefoperazone and ceftazi-dime in patients with cystic fibrosis. J Clin Hosp Pharm 1984; 9: 303–9.
Bosso JA, Saxon BA, Herbst JJ, et al. Azlocillin pharmacokinetics in patients with cystic fibrosis. Antimicrob Agents Chemother 1984; 25: 630–2.
Malmborg AS, Alfredsson H, Kusoffsky E, et al. Azlocillin and gentamicin in respiratory tract infections with Pseudomonas aeruginosa in patients with cystic fibrosis. Scand J Infect Dis 1981; 29 Suppl.: 64–9.
Prince AS, Neu HC. Use of piperacillin, a semisynthetic penicillin, in the therapy of acute exacerbations of pulmonary disease in patients with cystic fibrosis. J Pediatr 1980; 97: 148–51.
Van den Hollander JG, Overbeek SE, Vinks AATMM, et al. Pharmacokinetics of piperacillin and tazobactam during continuous and intermittent infusion in patients with cystic fibrosis [abstract]. Proceedings of the 26th International Congress on Chemotherapy; 1997 Jun 29–Jul 3; Sydney, 72–3.
Jacobs RF, Trang JM, Kearns GL, et al. Ticarcillin/clavulanic acid pharmacokinetics in children and young adults with cystic fibrosis. J Pediatr 1985; 106: 1001–7.
De Groot R, Hack BD, Weber A, et al. Pharmacokinetics of ticarcillin in patients with cystic fibrosis: a controlled prospective study. Clin Pharmacol Ther 1990; 47: 73–8.
Kercsmar CM, Stern RC, Reed MD, et al. Ceftazidime in cystic fibrosis: pharmacokinetics and therapeutic response. J Antimicrob Chemother 1983; 12 Suppl. A: 289–95.
Strandvik B, Malmborg AS, Alfredson H, et al. Clinical results and pharmacokinetics of ceftazidime treatment in patients with cystic fibrosis. J Antimicrob Chemother 1983; 12 Suppl. A: 283–7.
Padoan R, Brienza A, Crossignani RM, et al. Ceftazidime in treatment of acute pulmonary exacerbations in patients with cystic fibrosis. J Pediatrics 1983; 103: 320–4.
Permin H, Koch C, Høiby N, et al. Ceftazidime treatment of chronic Pseudomonas aeruginosa respiratory tract infection in cystic fibrosis. J Antimicrob Chemother 1983; 12 Suppl. A: 313–23.
Leeder SJ, Spino M, Isles AF, et al. Ceftazidime disposition in acute and stable cystic fibrosis. Clin Pharmacol Ther 1984; 36: 355–62.
Turner A, Pedler SJ, Carswell F, et al. Serum and sputum concentrations of ceftazidime in patients with cystic fibrosis. J Antimicrob Chemother 1984; 14: 521–7.
Mouton JW, Horrevorts AM, Overbeek SE, et al. Pharmacokinetics of ceftazidime during continuous and intermittent infusion in adult cystic fibrosis patients. In: Mouton JW, editor. Pharmacokinetic and pharmacodynamic studies of betalactam antibiotics in volunteers and patients with cystic fibrosis [thesis]. Rotterdam: Univ. of Rotterdam, 1993.
Vinks AATMM, Touw DJ, Heijerman HGM, et al. Pharmacokinetics of ceftazidime in adult cystic fibrosis patients during continuous infusion and ambulatory treatment at home. Ther Drug Monit 1994; 16: 341–8.
Reed MD, Stern RC, Yamashita TS, et al. Single-dose pharmacokinetics of cefsulodin in patients with cystic fibrosis. Antimicrob Agents Chemother 1984; 25: 579–81.
Hedman A, Alvan G, Strandvik B, et al. Increased renal clearance of cefsulodin due to higher glomerular filtration rate in cystic fibrosis. Clin Pharmacokinet 1990; 18: 168–75.
Arguedas AG, Stutman HR, Zaleska M, et al. Cefepime: pharmacokinetics and clinical response inpatients with cystic fibrosis. Am J Dis Child 1992; 146: 797–802.
Huls CE, Prince RA, Seilheimer DK, et al. Pharmacokinetics of cefepime in cystic fibrosis patients. Antimicrob Agents Chemother 1993; 37: 1414–6.
Hamelin BA, Moore N, Knupp CA, et al. Cefepime pharmacokinetics in cystic fibrosis. Pharmacotherapy 1993; 13: 465–70.
Reed MD, Stern RC, O’Brien C, et al. Pharmacokinetics of imipenem and cilastatin in patients with cystic fibrosis. Antimicrob Agents Chemother 1985; 27: 583–8.
Bergan T, Michalsen H, Malmborg AS, et al. Pharmacokinetic evaluation of imipenem combined with cilastatin in cystic fibrosis. Chemotherapy 1993; 39: 369–73.
Bins JW, Mattie H. Saturation of the tubular excretion of 3- lactam antibiotics. Br J Clin Pharmacol 1988; 25: 41–50.
Goldfarb J, Wormser GP, Inchiosa MA, et al. Single-dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. J Clin Pharmacol 1986; 26: 222–6.
Smith MJ, White LO, Bowyer H, et al. Pharmacokinetics and sputum penetration of ciprofloxacin in patients with cystic fibrosis. Antimicrob Agents chemother 1986; 30: 614–6.
LeBel M, Bergeron MG, Vallee F, et al. Pharmacokinetics and pharmacodynamics of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother 1986; 30: 260–6.
Stutman HR, Shalit I, Marks MI, et al. Pharmacokinetics of two dosage regimens if ciprofloxacin during a two-week therapeutic trial in patients with cystic fibrosis. Am J Med 1987; 82 Suppl. 4A: 142–5.
Davis RL, Koup JR, Williams-Warren J, et al. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrob Agents Chemother 1987; 31: 915–9.
Christensson BA, Nilsson-Ehle I, Ljungberg B, et al. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrob Agents Chemother 1992; 36: 2512–7.
Schaefer HG, Stass H, Wedgwood J, et al. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother 1996; 40: 29–34.
Rubio TT, Miles MV, Lettieri JT, et al. Pharmacokinetic disposition of sequential intravenous/oral ciprofloxacin in pediatric cystic fibrosis patients with acute pulmonary exacerbation. Pediatr Infect Dis J 1997; 16: 112–7.
Pleasant RA, Michalets EL, Williams DM, et al. Pharmacokinetics of vancomycin in adult cystic fibrosis patients. Antimicrob Agents Chemother 1996; 40: 186–90.
Boeckh M, Lode K, Borner K, et al. Pharmacokinetics and serum bactericidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob Agents Chemother 1988; 32: 92–5.
Healy DP, Polk RE, Garson ML, et al. Comparison of steadystate pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother 1987; 31: 393–7.
Ensom MHH, Davis GA, Cropp CD, et al. Clinical pharmacokinetics in the 21st century. Clin Pharmacokinet 1998; 34: 265–79.
Hyatt JM, McKinnon PS, Zimmer G, et al. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Clin Pharmacokinetic 1995; 28: 143–60.
Highet VS, Ballow CH, Forrest A. Population-derived AUIC is predictive of efficacy [abstract]. 95th American Society for Clinical Pharmacology and Therapeutics; 1994 Mar 30–Apr 1; New Orleans.
Horrevorts AM, De Witte J, Degener JE, et al. Tobramycin in patients with cystic fibrosis: adjustment in dosing interval for effective treatment. Chest 1987; 92: 844–8.
Winnie GB, Cooper JA, Witson J, et al. Comparison of 6 and 8 hourly tobramycin dosing intervals in treatment of pulmonary exacerbations in cystic fibrosis patients. Pediatr Infect Dis J 1991; 10: 381–6.
Guglielmo BJ, Quan LA, Stulbarg MS. Pharmacokinetics of once-daily versus thrice daily tobramycin in cystic fibrosis patients. J Antimicrob Chemother 1996; 37: 1040–2.
Smith DL, Stableforth DE, Geddes AM. Evaluation of a oncedaily netilmicin regimen in the treatment of cystic fibrosis. J Antimicrob Chemother 1994; 33: 191–3.
Phillips J, Nelson L, Kramer J, et al. Twelve hour pharmacokinetics and ototoxicity of tobramycin in CF [abstract. Pediatr Pulmonol 1997; 14 Suppl.: 263.
Wood PJ, Ioannides-Demos LL, Li SC, et al. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax 1996; 51: 369–73.
Samaniego-Picota MD, Whelton A. Aminoglycoside-induced nephrotoxicity in cystic fibrosis: a case presentation and review of the literature. Am J Ther 1996; 3: 248–57.
McRorie TI, Bosso J, Randolph L. Aminoglycoside ototoxicity in cystic fibrosis. Am J Dis Child 1989; 143: 1328–32.
Mulherin D, Fahy J, Grant W, et al. Aminoglycoside induced ototoxicity in patients with cystic fibrosis. Irish J Med Sci 1991; 160: 173–5.
Craig WA, Ebert SC. Continuous infusion of β-lactam antibiotics. Antimicrob Agents Chemother 1992; 36: 2577–83.
Mouton JW, Vinks AATMM. Is continuous infusion of β-lactam antibiotics worthwhile? Efficacy and pharmacokinetic considerations. J Antimicrob Chemother 1996; 38: 5–15.
Vogelman B, Craig WA. Kinetics of antibacterial activity. J Pediatr 1986; 108: 835–40.
Nicolau DP, Nightingale CH, Banevicius MA, et al. Serum bactericidal activity of ceftazidime: continuous infusion versus intermittent injections. Antimicrob Agents Chemother 1996; 40: 61–4.
Mouton JW, Den Hollander JG. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 1994; 38: 931–6.
Munzenberger PJ, Man-Ching J, Holliday SJ. Relationship of ceftazidime pharmacokinetic induces with the therapeutic outcome in patients with cystic fibrosis. Pediatr Infect Dis J 1993; 12: 997–1001.
Vinks AATMM, Brimicombe RW, Heijerman IGM, et al. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome, microbiology and pharmacokinetics. J Antimicrob Chemother 1997; 40: 125–33.
Schentag JJ, Nix DE, Adelman MH. Mathematical examination of dual individualization principles (1): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP Ann Pharmacother 1991; 25: 1050–7.
Forrest A, Nix DE, Bellow Ch, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously illpatients. Antimicrob Agents Chemother 1993; 37: 1073–81.
Vinks AATMM, Evers NAEM, Mathot RAA, et al. Impact of goal-oriented model-based TDM of aminoglycosides on clinical outcome: a cost-effectiveness analysis [abstract. Ther Drug Monit 1997; 19: 547.
Thomassen MJ, Demko CA, Doershuk CF. Cystic fibrosis: a review of pulmonary functions and interventions. Pediatr Pulmonol 1987; 3: 334–51.
Touw DJ, Vinks AATMM, Jacobs F, et al. Creatinine clearance as predictor of tobramycin elimination rate in adult patients with cystic fibrosis. Ther Drug Monit 1996; 18: 562–9.
Touw DJ, Vinks AATMM, Neef C. Pharmacokinetic modelling of intravenous tobramycin in adolescent and adult patients with cystic fibrosis using a nonparametric expectation maximization (NPEM) algorithm. Pharm World Sci 1997; 19: 142–51.
Touw DJ, Vinks AATMM, Neef C. Comparative evaluation of a 1- and 2-compartment model for i.v. tobramycin in adult patients with cystic fibrosis [abstract]. Ther Drug Monit 1997; 19: 570.
Bosso J, Relling MV, Townsend PL, et al. Intrapatient variation in aminoglycoside disposition in cystic fibrosis. Clin Pharmacol 1987; 6: 54–8.
Delage G, Desautels L, Legault S, et al. Individualized aminoglycoside dosage regimens in patients with cystic fibrosis. Drug Intell Clin Pharm 1988; 22: 386–9.
Jelliffe RW, Schumitzky A, Bayard D, et al. Model-based, goaloriented, individualised drug therapy: linkage of population modelling, a new ‘multiple model’ dosage design, Bayesian feedback and individualised target goals. Clin Pharmacokinet 1998; 34: 57–77.
Jelliffe RW, Iglesias T, Hurst AK, et al. Individualising gentamicin dosage regimens. A comparative review of selected models, data fitting methods and monitoring strategies. Clin Pharmacokinet 1991; 21: 461–78.
Hoigne R, Neftel K, Cerny A, et al. Penicillins, cephalosporins and other beta-lactam antibiotics. In: Dukes MNG, editor. Meyler’s side effects of drugs. 12th ed. Amsterdam: Elsevier Science Publishers B.V, 1992: 593–9.
Vinks AATMM, Mouton JW, Touw DJ, et al. Population pharmacokinetics of ceftazidime in cystic fibrosis patients analyzed using a nonparametric algorithm and optimal sampling strategy. Antimicrob Agents Chemother 1996; 40: 1091–7.
Mouton JW, Vinks AATMM, Punt NC. Pharmacokinetic-pharmacodynamic modelling of activity of ceftazidime during continuous infusion and intermittent infusion. Antimicrob Agents Chemother 1997; 41: 733–8.
Maire P, Barbaut X, Vergnaud JM, et al. Computation of drug concentrations in endocardial vegetation in patients during antibiotic therapy. Int J Biomed Comput 1994; 36: 77–85.
Bouvier d’Yvoire MJY, Maire PH. Dosage regimens of antibacterials. Implications of a pharmacokinetic/pharmacodynamic model. Clin Drug Invest 1996; 11: 229–39.
Mouton JW, Vinks AATMM, Punt N, et al. Pharmacokinetic pharmacodynamic modelling of bacterial killing in vivo [abstract]. Proceedings of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sep 28–Oct 1: Toronto, 5.
Levy J. Antibiotic activity in sputum. JPediatr 1986; 108: 841–6.
Jelliffe RW, Schumitzky A, Van Guilder M, et al. User manual for version 10.7 of the USC*PACK collection of PC programs. Laboratory of Applied Pharmacokinetics, Univ. of Southern California, School of Medicine, Los Angeles; 1996 Feb.
Bollister N, Basker M, Hodges NA, et al. The diffusion of 3- lactam antibiotics through mixed gels of cystic fibrosis-derived mucin and Pseudomonas aeruginosa alginate. J Antimicrob Chemother 1991; 27: 667–74.
Gordon CA, Hodges NA, Mariott C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother 1988; 22: 667–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Touw, D.J., Vinks, A.A.T.M.M., Mouton, J.W. et al. Pharmacokinetic Optimisation of Antibacterial Treatment in Patients with Cystic Fibrosis. Clin Pharmacokinet 35, 437–459 (1998). https://doi.org/10.2165/00003088-199835060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199835060-00003