Abstract
Objectives: Adverse drug reactions (ADRs) leading to hospitalisation or occurring during hospital stay contribute significantly to patient morbidity and mortality as well as representing an additional cost for healthcare systems. The aim of this study was to provide data about the type and incidence of ADRs in a neurological department and to compare two different methodological approaches to collecting information on ADRs.
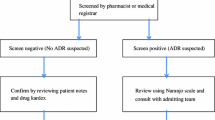
Methods: The two methods used were intensified surveillance of neurological wards by daily ward rounds and computer-assisted screening for ADRs by means of pathological laboratory parameters.
Results: Of admissions to the neurological department, 2.7% were caused by an ADR and 18.7% of patients experienced at least one ADR during hospitalisation. The positive predictive values of pathological laboratory parameters ranged between 0% (eosinophil count) and 100% for increased drug serum concentrations and international normalised ratio, i.e. the latter were always accompanied by a clinically relevant ADR. However, only half of all ADR could be detected by pathological laboratory parameters, the sensitivity of this method came to 45.1% with a specificity of 78.9%.
Conclusion: Similar to departments of internal medicine, a high number of ADRs occur on neurological wards. The predominant ADRs were those typical of neurotropic medications such as dyskinesia and increased sedation. Due to the age of the patients involved, cardiovascular co-medication is often prescribed and represents an additional risk factor for ADRs. By measuring pathological laboratory parameters the majority of ADRs could not be detected in neurological patients.






Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA 1998; 279(15): 1200–17
Mannesse CK, Derkx FHM, de Ridder MAJ, et al. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing 2000; 29(1): 35–9
Hallas J, Gram LF, Grodum E, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol 1992; 33(1): 61–8
Roughead EE, Gilbert AL, Primrose JG, et al. Drug-related hospital admissions: a review of Australian studies published 1988-1996. Med J Aust 1998; 168(8): 405–8
Muehlberger N, Schneeweiss S, Hasford J. Adverse drug reaction monitoring: cost and benefit considerations part I: frequency of adverse drug reactions causing hospital admission. Pharmacoepidemiol Drug Saf 1997; 6Suppl. 3: 71S–77S
Pouyanne P, Haramburu F, Imbs JL, et al. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ 2000; 320: 1036
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients: excess length of stay, extra cost, and attributable mortality. JAMA 1997; 277(4): 301–6
Dormann H, Muth-Selbach U, Krebs S, et al. Incidence and costs of adverse drug reactions during hospitalisation. Drug Saf 2000; 22(2): 161–8
Bates DW, Spell N, Cullen DC, et al. The costs of adverse drug events in hospitalized patients. JAMA 1997; 277(4): 307–11
Moore N, Lecointre D, Noblet C, et al. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol 1998; 45(3): 301–8
Azaz-Livshits T, Levy M, Sadan B, et al. Computerized surveillance of adverse drug reactions in hospital: pilot study. Br J Clin Pharmacol 1998; 45(3): 309–14
Fattinger K, Roos M, Vergères P, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 2000; 49(2): 158–67
Imbs JL, Pouyanne P, Haramburu F, et al. Iatrogénie médicamenteuse: estimation de sa prévalence dans les hôpitaux publics français. Therapie 1999; 54(1): 21–7
Bennett CL, Connors JM, Carwil JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342(24): 1773–7
Wood AJ. Thrombotic thrombocytopenic purpura and clopidogrel: a need for new approaches to drug safety. N Engl J Med 2000; 342(24): 1824–6
Landis NT. ADE rate uncertain, reporting systems inadequate, GAO tells legislators. Am J Health Syst Pharm 2000; 57(6): 515–9
Currie CJ, MacDonald TM. Use of routine healthcare data in safe and cost-effective drug use. Drug Saf 2000; 22(2): 97–102
van den Bemt PMLA, Egberts ACG, Lenderink AW, et al. Adverse drug events in hospitalised patients: a comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol 1999; 55(2): 155–8
Gardner RM, Pryor TA, Warner HR. The HELP hospital information system: update 1998. Int J Med Inf 1999; 54(3): 169–82
Classen DC, Pestotnik SL, Evans RS, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991; 266(20): 2847–51
Francis GS. Cardiac complications in the intensive care unit. Clin Chest Med 1999; 20(2): 269–85
Impicciatore P, Choonara I, Clarkson A, et al. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol 2001; 52(1): 77–83
World Health Organisation. International drug monitoring: the role of national centres.WHO Tech Rep Series 1972, No 498
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, andmanagement. Lancet 2000; 356(9237): 1255–9
Rawlins MR. Clinical Pharmacology: adverse reactions to drugs. BMJ 1981; 282(6268): 974–6
Herings RMC, de Boer A, Stricker BHC, et al. Hypoglycaemia associated with the use of inhibitors of angiotensin converting enzyme. Lancet 1995; 345(8959): 1195–8
Schneeweiss S, Göttler M, Hasford J, et al. First results from an intensified monitoring system to estimate drug-related hospital admissions. Br J Clin Pharmacol 2001; 52(2): 196–200
Klotz U. Effect of age on pharmacokinetics and pharmacodynamics in man. Clin Pharmacol Ther 1998; 36(11): 581–5
Thürmann PA, Hompesch BC. Influence of gender on pharmacokinetics and pharmacodynamics of drugs. Int J Clin Pharmacol Ther 1998; 36(11): 586–90
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. JAMA 1995; 274(1): 29–34
Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999; 282(3): 267–70
Grohmann R, Hippius H, Müller-Oehrlinghausen B, et al. Assessment of adverse drug reactions in psychiatric hospitals. Eur J Clin Pharmacol 1984; 26(6): 727–34
Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol 1990; 11(2): 272–6
Hall M, McCormack P, Arthurs N, et al. The spontaneous reporting of adverse drug reactions by nurses. Br J Clin Pharmacol 1995; 40(2): 173–5
Acknowledgements
We are indebted to all physicians and nurses in the department of neurology during our data collection period and especially Professor Joerg, MD for his continuous advice. We also thank S. Pasche for his technical assistance and Dr C. Zwernemann for his support in data collection.
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thuermann, P.A., Windecker, R., Steffen, J. et al. Detection of Adverse Drug Reactions in a Neurological Department. Drug-Safety 25, 713–724 (2002). https://doi.org/10.2165/00002018-200225100-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200225100-00004