Abstract
Introduction: Since April 1997, UK hospital pharmacists have been invited to submit reports of suspected adverse drug reactions (ADRs) to the Committee on Safety of Medicines (CSM) and Medicines Control Agency. Three studies have investigated the involvement of hospital pharmacists in ADR reporting; however, they did not investigate the possible factors that could affect ADR reporting.
Objectives: (i) To analyse the extent to which hospital pharmacists think that specified factors could affect reporting ADRs; (ii) to identify any additional factors that could hinder reporting; and (iii) to recommend possible methods to improve reporting.
Methods: Piloted questionnaires were sent to 548 hospital pharmacists in Great Britain randomly selected by the Royal Pharmaceutical Society of Great Britain (RPSGB) from their computer database. 346 questionnaires were returned and 280 were included in this study.
Results: 46% of the pharmacists had identified ADRs that were considered to be reportable according to the CSM criteria in the 6 months prior to the survey. 39% did not report these ADRs either to the CSM or the manufacturers. Only 8.2% reported that their hospitals had a written policy; conversely, 73.7% agreed that such a policy could enhance ADR reporting. Although not statistically significant, the result showed an increasing tendency to report ADRs by pharmacists who had received training. Furthermore, there was an increasing tendency to report ADRs with increasing seniority.
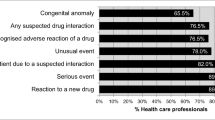
Discussion: The results show that hospital pharmacists say they are more likely to report serious and rare ADRs and ADRs associated with newly marketed drugs. Factors that could reduce ADR reporting included being busy at work, lack of confidence in recognising ADRs and the fear of breaching patient confidentiality. Most common suggestions on methods to improve ADR reporting were to provide ADR training and meetings (34%) and a hospital written policy (24%).
Recommendations: ADR training and meetings would be a useful step in improving hospital pharmacist ADR reporting. Therefore, we recommend that the CSM and the RPSGB liaise with regional drug information centres and schools of pharmacy to provide more study days and training programmes for hospital pharmacists. Furthermore, the CSM should write to the ‘Drugs and Therapeutics Committee’ of each hospital and encourage them to develop a written local policy for pharmacist ADR reporting. Further studies should be conducted to test the recommendations noted here, assessing the response of the pharmacists in terms of absolute numbers of reports made. It would be particularly interesting to study the need for a written hospital policy and education.






Similar content being viewed by others
References
Lazaron J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalised patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–5
Pirmohamed M, Breckenridge AM, Kitteringham NR, et al. Clinical review. Adverse drug reactions. BMJ 1998; 316: 1295–8
Wong ICK. Pharmacovigilance resources in the United Kingdom. Pharm J 1999; 263: 285–8
Smith J. Have your say in ADR reporting. Pharmacy in Practice 1997; 7 465
Calvert RT. Clinical pharmacy — a hospital perspective. Br J Clin Pharmacol 1999; 47: 231–8
Lee A, Bateman DN, Edwards C, et al. Reporting of adverse drug reactions by hospital pharmacists: pilot scheme. BMJ 1997; 315: 519
Anonymous. Pharmacist’s adverse drug reaction reporting to start on April 1. Pharm J 1998; 258: 330–1
Green CF, Mottram DR, Rowe P, et al. An investigation into adverse drug reaction monitoring by United Kingdom hospital pharmacy departments. Int J Pharm Pract 1997; 5: 202–8
Anonymous. Hospital pharmacist ‘Yellow Card’ reporting found to be ‘insufficiently developed’. Pharm J 1998; 261: 44
Davis S, Coulson RA, Wood SM. Adverse drug reaction reporting by hospital pharmacists: the first year. Pharm J 1999; 262: 366–7
Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol 1995; 39: 223–6
Talbot JC. Spontaneous reporting. In: Glaxo Research Group, editors. Drug safety - a shared responsibility. New York (NY): Churchill Livingstone, 1991; 38
Ferguson M, Dhillon S. A survey of adverse drug reaction reporting by hospital pharmacists to the committee on safety of medicines — the role of pharmacy departments. Int J Pharm Pract 1999; 7(3): 167–71
Committee on Safety of Medicines, Medicines Control Agency. Pharmacovigilance — the Yellow Card Scheme. Information pack for pharmacists, 1997
Anonymous. Yellow card reporting - how far have we come? Hosp Pharm 2000; 7: 103–6
Green CF, Mottram DR, Pimohamed M, et al. A record year for the ‘green card’ adverse drug reaction reporting scheme. UKCPA Symposium. An investigation into adverse drug reaction reporting by hospital pharmacy departments in the United Kingdom. 1997 Nov 21, Blackpool
Acknowledgements
We would like to thank all the hospital pharmacists who participated in this study and Dr David Wright and Mr Jon Silcock for their advice regarding the design of the questionnaire. We also thank Professor Henry Chrystyn for his helpful comments. Dr Ian Wong is a former Scientific Officer at the Medicines Control Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sweis, D., Wong, I.C. A Survey on Factors that Could Affect Adverse Drug Reaction Reporting According to Hospital Pharmacists in Great Britain. Drug-Safety 23, 165–172 (2000). https://doi.org/10.2165/00002018-200023020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200023020-00006