Abstract
The safety of drug therapy for inflammatory bowel disease during pregnancy is an important clinical concern. Current available information is largely derived from animal studies and clinical experience among patients with inflammatory bowel disease and autoimmune disorders and organ transplant recipients. However, these data are confounded by various factors including difficulty projecting the results of animal studies to humans, methodological deficiencies of some studies, insufficient experience with certain agents, difficulty distinguishing the fetal effects of underlying disease from drug therapy and a need to consider the impact of background rates of adverse fetal outcomes which apply to all pregnancies.
In inflammatory bowel disease, the effects of active inflammation on the fetus are believed to be more harmful than those of drug treatment, and therapy is often justified to induce or maintain remission during pregnancy. The choice of appropriate treatment is determined by the severity of the disease and the potential for drug toxicity.
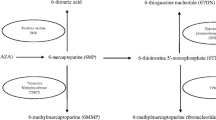
No causal relationship has been established between exposure to sulfasalazine or other 5-aminosalicylic acid drugs and the development of congenital malformations. These drugs may be used with relative safety during pregnancy and lactation. Considerable experience with corticosteroids have shown them to pose very small risk to the developing fetus. Current evidence indicates that maternal use of azathioprine is not associated with an increased risk of congenital malformations, though impaired fetal immunity, growth retardation or prematurity is occasionally observed. Preliminary evidence derived from patients with inflammatory bowel disease show no significant fetal toxicity following first trimester exposure to mercaptopurine, though its elective use in pregnancy is controversial. Cyclosporin is not teratogenic, but may be associated with growth retardation and prematurity. Pregnancy should be avoided in women treated with methotrexate because of its known abortifacient effects and risk of causing typical malformations. Although treatment with metronidazole or ciprofloxacin for short durations appear to be devoid of adverse fetal reactions, the effect of prolonged exposure as required in Crohn’s disease remains unknown.
Similar content being viewed by others
References
Hudson M, Flett G, Sinclair TS, et al. Fertility and pregnancy in inflammatory bowel disease. Int J Gynecol Obstet 1997; 58(2): 222–37
Baird DD, Narendranathan M, Sandler RS. Increased risk of preterm birth for women with inflammatory bowel disease. Gastroenterology 1990; 99(4): 987–94
Mayberry JF, Weterman IT. European survey of fertility and pregnancy in women with Crohn’s disease: a case control study by European collaborative group. Gut 1986; 27(7): 821–5
Willoughby CP, Truelove SC. Ulcerative colitis and pregnancy. Gut 1980; 21: 469–74
Woolfson K, Cohen Z, McLeod RS. Crohn’s disease and pregnancy. Dis Colon Rectum 1990; 33(10): 869–73
Hanan IM, Kirsner JB. Inflammatory bowel disease in the pregnant woman. Clin Perinatol 1985; 12: 682–99
Miller JP. Inflammatory bowel disease in pregnancy: a review. J R Soc Med 1986; 79: 221–5
Modigliani R. Drug therapy for ulcerative colitis during pregnancy. Eur J Gastroenterol Hepatol 1997; 9(9): 854–7
Khosla R, Willoughby CP, Jewell DP. Crohn’s disease and pregnancy. Gut 1984; 25(1): 52–6
Mogadam M, Dobbins WO, Korelitz BI, et al. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterology 1981; 80(1): 72–6
Baiocco PJ, Korelitz BI. The influence of inflammatory bowel disease and its treatment on pregnancy and fetal outcome. J Clin Gastroenterol 1984; 6(3): 211–6
Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998; 338: 1128–37
Truelove SC, Witts LJ. Cortisone in ulcerative colitis. BMJ 1955; II: 1041–8
Summers RW, Switz DM, Sessions JT, et al. The National cooperative Crohn’s disease study: results of drug treatment. Gastroenterology 1979; 77: 847–69
Kjeldsen J. Treatment of ulcerative colitis with high doses of oral prednisolone: the rate of remission, the need for surgery, and the effect of prolonging the treatment. Scand J Gastroenterol 1993; 28(9): 821–6
Malchow H, Ewe K, Brandes JW, et al. European cooperative Crohn’s disease study (ECCDS): results of drug treatment. Gastroenterology 1984; 86(2): 249–66
Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 1994; 35(3): 360–2
Esplin MS, Branch DW. Immunosuppressive drugs and pregnancy. Obstet Gynecol Clin North Am 1997; 24(3): 601–16
Albengres E, Le Louet H, Tillement JP. Immunosuppressive drugs and pregnancy: experimental and clinical data. Transplant Proc 1997; 29: 2461–6
Pinski L, DiGeorge AM. Cleft palate in the mouse: a teratogenic index of glucocorticoid potency. Science 1965; 147: 402–3
Walker BE. Induction of cleft palate in rabbits by several glucocorticoids. Proc Soc Exp Biol Med 1967; 125: 1281–4
Ballard PD, Hearney EF, Smith MB. Comparative teratogenicity of selected glucocorticoids applied ocularly in mice. Teratology 1977; 16: 175–80
Rayburn WF. Glucocorticoid therapy for rheumatic diseases: maternal, fetal, and breast-feeding considerations. Am J Reprod Immunol 1992; 28(3–4): 138–40
Kihlstrom I, Lundberg C. Teratogenicity study of the new glucocorticosteroid budesonide in rabbits. Arzneimittel Forschung 1987; 37(1): 43–6
Beitins IZ, Bayard F, Ances IG, et al. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone near term. Pediatr Res 1973; 7: 509–19
Blanford AT, Murphy BEP. In vitrometabolism of prednisolone, dexamethasone, betamethasone, and cortisol by the human placenta. Am J Obstet Gynecol 1977; 127: 264–7
Kenny MJ, Preeyasombat C, Spaudling JS, et al. Cortisol production rate in infants born of steroid-treated mothers and of diabetic mothers. Pediatrics 1966; 37(6): 960–6
Harris JWS, Ross IP. Cortisone therapy in early pregnancy: relation to cleft palate. Lancet 1956; I: 1045–7
Schatz M. Asthma treatment during pregnancy: what can be safely taken? Drug Saf 1997; 16(5): 342–50
Fraser FC, Sajoo A. Teratogenic potential of corticosteroids in humans. Teratology 1995; 51(1): 45–6
Reinisch JM, Simon NG, Karow WG, et al. Prenatal exposure to prednisolone in humans and animals retards intrauterine growth. Science 1978; 202(4366): 436–8
Scott JR. Fetal growth retardation associated with maternal administration of immunosuppressive drugs. Am J Obstet Gynecol 1977; 128(6): 668–76
Warrell DW, Taylor R. Outcomes for the fetus of mothers receiving prednisolone during pregnancy. Lancet 1968; I: 117–8
Bongiovanni AM, McPadden AJ. Steroids during pregnancy and possible fetal consequences. Fertil Steril 1960; 11: 181–6
Bulmash JM. Systemic lupus erythematosus and pregnancy. Obstet Gynecol Annu 1978; 7: 153–94
Turner ES, Greenberger PA, Patterson R. Management of the pregnant asthmatic patient. Ann Int Med 1980; 93: 905–18
Katz FH, Duncan BR. Entry of prednisolone into breast milk [letter]. N Engl J Med 1975; 293: 1154
Ost L, Wettrell G, Bjorkhem I, et al. Prednisolone excretion into breast milk. J Pediatr 1985; 106(6): 1008–11
Greenberger PA, Odeh YK, Frederiksen MC, et al. Pharmacokinetics of prednisolone transfer to breast milk. Clin Pharmacol Ther 1993; 53(3): 324–8
Klotz U, Maier K, Fischer C, et al. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn’s disease. N Engl J Med 1980; 303: 1499–502
Peppercorn MA. Sulfasalazine. Pharmacology, clinical use, toxicity, and related drug developments. Ann Intern Med 1984; 101: 377–86
O’Morain C, Smethurst P, Dore CJ, et al. Reversible male infertility due to sulphasalazine: studies in man and rat. Gut 1984; 25: 1078–84
Craxi A, Pagliarello F. Possible embryotoxicity of sulfasalazine. Arch Intern Med 1980; 140(12): 1674
Newman NM, Correy JF. Possible teratogenicity of sulphasalazine. Med J Aust 1983; 1(11): 528–9
Hoo JJ, Hadro TA, Von Behren P. Possible teratogenicity of sulfasalazine [letter]. N Engl J Med 1988; 318: 1128
Levi S, Liberman M, Levi AJ, et al. Reversible congenital neutropenia associated with maternal sulphasalazine therapy. Eur J Pediatr 1988; 148(2): 174–5
Moody AG, Probert C, Jayanthi V, et al. The effects of chronic ill health and treatment with sulphasalazine on fertility amongst men and women with inflammatory bowel disease in Leicestershire. Int J Colorectal Dis 1997; 12: 220–4
Willoughby CP. Fertility, pregnancy and inflammatory bowel disease. In: Allan RN, Keighley MRB, Hawkins CF, et al., editors. Inflammatory bowel disease, 2nd ed. Edinburgh: Churchill Livingstone, 1990: 547–58
Nielsen OH, Andreasson B, Bondesen S, et al. Pregnancy in ulcerative colitis. Scand J Gastroenterol 1983; 18: 735–42
Hensleigh PA, Kauffman RE. Maternal absorption and placental transfer of sulphasalazine. Am J Obstet Gynecol 1977; 127(4): 443–4
Esbjorner E, Jarnerot G, Wranne L. Sulphasalazine and sulphapyridine serum levels in children to mothers treated with sulphasalazine during pregnancy and lactation. Acta Paediatr Scand 1987; 76(1): 137–42
Jarnerot G, Into-Malmberg MB, Esbjorner E. Placental transfer of sulphasalazine, sulphapyridine and some of its metabolites. Scand J Gastroenterol 1981; 16: 693–7
Azad Khan AK, Truelove SC. Placental and mammary transfer of sulphasalazine. BMJ 1979; 2: 1553
Czeizel AE, Dundas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992; 327(26): 1832–5
Connell WR. Safety of drug therapy for inflammatory bowel disease in pregnant and nursing women. Inflamm Bowel Dis 1996; 2: 33–47
Jarnerot G, Into-Malmberg MB. Sulphasalazine treatment during breast feeding. Scand J Gastroenterol 1979; 14: 869–71
Branski D, Kerem E, Gross-Kieselstein E, et al. Bloody diarrhea possible complication of sulfasalazine transferred through human breast milk. J Pediatr Gastroenterol Nutr 1986; 5(2): 316–7
Committee on Drugs, American Academy of Paediatrics. The transfer of drugs and other chemicals into human milk. Paediatrics 1994 93(1): 137–50
Sutherland LR, May GR, Shaffer EA. Sulfasalazine revisited: a meta-analysis of 5 amino salicylic acid in the treatment of ulcerative colitis. Ann Intern Med 1993; 118: 540–9
Mulder CJJ, Tytgat GNJ, Wetermen T, et al. Double-blind comparison of slow release 5-aminosalicylate and sulfasalazine in remission maintenance in ulcerative colitis. Gastroenterology 1988; 95: 1449–53
Singleton JW, Hanauer SB, Gitnick GL, et al. Mesalazine capsules for the treatment of active Crohn’s disease: results of a 16-week trial. Gastroenterology 1993; 104: 1293–301
Messori A, Brignola C, Trallori G, et al. Effectiveness of 5-aminosalicylic acid for maintaining remission in patients with Crohn’s disease: a meta-analysis. Am J Gastroenterol 1994; 89: 692–8
Marteau P, Nelet F, Le Lu M, et al. Adverse events in patients treated with 5-aminosalicylic acid: 1993–1994 pharmacovigilance report for Pentasa in France. Aliment Pharmacol Ther 1996; 10: 949–56
Meyers S, Sachar DB, Present DH, et al. Olsalazine in the treatment of ulcerative colitis among patients intolerant to sulfasalazine: a prospective, randomised, placebo-controlled. double-blind, dose-ranging clinical trial. Scand J Gastroenterol 1988; 23: 29–37
Diav-Citrin O, Park YH, Veerasuntharam G, et al. The safety of mesalamine in human pregnancy: a prospective controlled cohort study. Gastroenterology 1998; 114: 23–8
Lundberg C, Asberg I, Smedegard G, et al. 5-aminosalicylic acid (5-ASA)-induced nephrotoxicity is dose dependent in rats [abstract]. Gastroenterology 1996; 112: A952
Marteau P, Devaux CB. Mesalazine during pregnancy. Lancet 1994; 344: 1708–9
Segars LW, Gales BJ. Mesalamine and olsalazine: 5-aminosalicylic acid agents for the treatment of inflammatory bowel disease. Clin Pharm 1992; 11(6): 514–28
Christensen LA, Rasmussen SN, Hansen SH. Disposition of 5-aminosalicylic acid and N-acetyl-5-aminosalicylic acid in fetal and maternal body fluids during treatment with different 5-aminosalicylic acid preparations. Acta Obstet Gynecol Scand 1994; 74(5): 399–402
Colombel J, Brabant G, Gubler M, et al. Renal insufficiency in infant: side-effect of prenatal exposure to mesalazine? Lancet 1994; 344: 620–1
Habal FM, Hui G, Greenberg GR. Oral 5-aminosalicylic acid for inflammatory bowel disease in pregnancy: safety and clinical course. Gastroenterology 1993; 105(4): 1057–60
Trallori G, d’Albasio G, Bardazzi G, et al. 5-Aminosalicylic acid in pregnancy: clinical report. Ital J Gastroenterol 1994; 26: 75–8
Bell CM, Habal FM. Safety of topical 5-aminosalicylic acid in pregnancy. Am J Gastroenterol 1997; 92(12): 2201–2
Klotz U, Harings-Kaim A. Negligible excretion of 5-aminosalicylic acid in breast milk. Lancet 1993; 342: 618–9
Nelis GF. Diarrhoea due to 5-aminosalicylic acid in breast milk [letter]. Lancet 1989; I: 383
Ito S, Blajchman A, Stephenson M, et al. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am J Obstet Gynecol 1993; 168: 1393–9
Ginsberg AL, Davis ND, Nochomovitz LE. Placebo-controlled trial of ulcerative colitis with oral 4-aminosalicylic acid Gastroenterology 1992; 102(2): 448–52
Schreiber S, Howaldt S, Raedler A. Oral 4-aminosalicylic acid versus 5-aminosalicylic acid slow release tablets: double blind, controlled pilot study in the maintenance treatment of Crohn’s ileocolitis. Gut 1994; 35(8): 1081–5
Present DH, Meltzer SJ, Krumholz MP, et al. 6-mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989; 111: 641–9
O’Donoghue DP, Dawson AM, Powell-Tuck J, et al. Doubleblind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet 1978; II: 955–7
Voogd CE. Azathioprine, a genotoxic agent to be considered non-genotoxic in man. Mutat Res 1989; 221(2): 133–52
Githens JH, Rosenkrantz JG, Tunnock SM. Teratogenic effects of azathioprine (Imuran). J Pediatr 1965; 66: 962–3
Rosenkrantz JG, Githens JH, Cox SM, et al. Azathioprine (Imuran) and pregnancy. Am J Obstet Gynecol 1967; 97: 387–94
Tuchmann-Duplessis H, Mercier-Parot L. Production in rabbits of malformations of the limbs by administration of azathioprine and 6-mercaptopurine. C R Seances Soc Biol Fil 1966; 166(3): 501–6
Bermas BL, Hill JA. Effects of immunosuppressive drugs during pregnancy. Arthritis Rheum 1995; 38(12): 1722–32
Reimers TJ, Sluss PM. 6-mercaptopurine treatment of pregnant mice: effects on second and third generations. Science 1978; 201: 65–7
Saarikoski S, Seppala M. Immunosuppression during pregnancy: transmission of azathioprine and its metabolites from the mother to the fetus. Am J Obstet Gynecol 1973; 115: 1100–6
Ramsey-Goldman R, Schilling E. Immunosuppressive drug use during pregnancy. Rheum Dis Clin North Am 1997; 23(1): 149–67
Alstead EM, Ritchie JK, Lennard-Jones JE, et al. Safety of azathioprine in pregnancy in inflammatory bowel disease. Gastroenterology 1990; 99(2): 443–6
Davison JM. Pregnancy in renal allograft recipients: prognosis and management. Ballieres Clin Obstet Gynaecol 1987; 1(4): 1027–45
Baxi LV, Rho RB. Pregnancy after cardiac transplantation. Am J Obstet Gynecol 1993; 169(1): 33–4
Haagsma EB, Visser GHA, Klompmaker IJ, et al. Successful pregnancy after orthotopic liver transplantation. Obstet Gynecol 1989; 74: 442–3
Ramsey-Goldman R, Mientus JM, Kutzer JE, et al. Pregnancy outcome in women with systemic lupus erythematosus treated with immunosuppressive drugs. J Rheum 1993; 20(7): 1152–7
Penn I, Makowski EL, Harris P. Parenthood following renal transplantation. Kidney Int 1980; 18: 221–33
Roubenoff R, Hoyt J, Petri M, et al. Effects of antiinflammatory immunosuppressive drugs on pregnancy and fertility. Semin Arthritis Rheum 1988; 18: 88–110
Whetham JCG, Cardella C, Harding M. Effect of pregnancy on graft function and graft survival in renal cadevar transplant patients. Am J Obstet Gynecol 1983; 145: 193–7
Wagoner LE, Taylor DO, Olsen SL, et al. Immunosuppressive therapy, management, and outcome of heart transplant recipients during pregnancy. J Heart Lung Transplant 1994; 13: 993–1000
Brown JH, Maxwell AP, McGeown MG. Outcome of pregnancy following renal transplantation. Ir J Med Sci 1991; 160: 255–6
Marushak A, Weber T, Bock J, et al. Pregnancy following kidney transplantation. Acta Obstet Gynaecol Scand 1986; 65: 557–9
O’Donnell D, Sevitz H, Seggie JL. Pregnancy after renal transplantation. Aust N Z J Med 1985; 15: 320–5
The Consultative Council on Obstetrics and Paediatric Mortality and Morbidity. Annual report for the year 1997: incorporating the 36th Survey of Perinatal Deaths in Victoria. Melbourne, 1998: 39
Pirson Y, van Lierde M, Ghysen J, et al. Retardation of fetal growth in patients receiving immunosuppressive therapy [letter]. N Engl J Med 1985; 313(5): 328
Little BB. Immunosuppressant therapy during gestation. Semin Perinatol 1997; 21(2): 143–8
Briggs GG, Freeman RK, Yaffe SJ, editors. Drugs in pregnancy and lactation. 4th ed. Baltimore (MA): Williams and Wilkins, 1994: 80
DeWitte DB, Buick MK, Cyran SE, et al. Neonatal pancytopenia and severe combined immunodeficiency associated with antenatal administration of azathioprine and prednisone. J Pediatr 1984; 105: 625–8
Cote CJ, Meuwissen HJ, Pickering RJ. Effects on the neonate of prednisolone and azathioprine administered to the mother during pregnancy. J Pediatr 1974; 85(3): 324–8
Davison JM, Dellagrammatikas H, Parkin JM. Maternal azathioprine therapy and depressed haemopoiesis in the babies of renal allograft patients. Br J Obstet Gynaecol 1985; 92: 233–9
Huynh LA, Min DI. Outcomes of pregnancy and the management of immunosuppressive agents to minimise fetal risks in organ transplant patients. Ann Pharmacotherapy 1994; 28: 1355–7
Ostrer H, Stamberg J, Perinchief P. Two chromosome aberrations in the child of a woman with systemic lupus erythematosus treated with azathioprine and prednisone. Am J Med Genet 1984; 17: 627–32
Leb DE, Weisskopf B, Kanovitz BS. Chromosome aberrations in the child of a kidney transplant recipient. Arch Int Med 1971; 128(3): 441–4
Francella A, Dayan A, Rubin P, et al. 6-mercaptopurine is safe therapy for child bearing patients with inflammatory bowel disease (IBD): a case controlled study [abstract]. Gastroenterology 1996; 112(4): A909
Marion JF. Toxicity of 6-mercaptopurine/azathioprine in patients with inflammatory bowel disease. Inflamm Bowel Dis 1998; 2: 116–7
Rajapakse RO, Korelitz BI, Zlatanic J, et al. Outcome of pregnancies when fathers are treated with 6-mercaptopurine for inflammatory bowel disease [abstract]. Gastroenterology 1998; 114: A1066
Grekas DM, Vasiliou SS, Lazarides AN. Immunosuppressive therapy and breast-feeding after renal transplantation [letter]. Nephron 1984; 37: 68
Lichtiger S, Present D, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841–5
Sandborn WJ. Areview of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol 1996; 91(3): 423–33
Ryffel B, Donatsch P, Madorin M, et al. Toxicological evaluation of cyclosporin A. Arch Toxicol 1983; 53: 107–41
Brown PAJ, Gray ES, Whiting PH, et al. Effects of cyclosporin A on fetal development in the rat. Biol Neonate 1985; 48: 172–80
Mason RJ, Thomson AW, Whiting PH, et al. Cyclosporine-induced fetotoxicity in the rat. Transplantation 1985; 39: 9–12
Al-Chalabai HA. Effect of cyclosporin A on the morphology and function of the ovary and fertility in the rabbit. Int J Fertil 1984; 29: 218–23
Seethalakashmi L, Flores C, Carboni AA, et al. Cyclosporine: its effects on testicular function and fertility in the prepubertal rat. J Androl 1990; 11: 17–24
Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 outcomes in transplant recipients treated with Sandimmun. Transplant Proc 1997; 29: 2480
Armenti VT, Ahlswede KM, Ahlswede BA, et al. National transplantation pregnancy registry: outcomes of 154 pregnancies in cyclosporin-treated female kidney transplant recipients. Transplantation 1994; 57(4): 502–6
Haugen G, Fauchald P, Sodal G, et al. Pregnancy outcome in renal allograft recipients: influence of ciclosporin A. Eur J Obstet Gynecol Reprod Biol 1991; 39: 25–9
Ostensen M. Treatment with immunosuppressive and disease modifying drugs during pregnancy and lactation. Am J Reprod Immunol 1992; 28: 148–52
Shaheen FAM, Al-Sulaiman MH, Al-Khader AA. Long-term nephrotoxicity after exposure to cyclosporine in utero. Transplantation 1993; 56(1): 224–5
Roll C, Luboldt HJ, Winter A, et al. Hepatoblastoma in a 2-year-old child of a liver-transplanted mother. Lancet 1997; 349: 103
Anderson JB, Turner GM, Williamson RCN. Fulminant ulcerative colitis in late pregnancy and the puerperium. J R Soc Med 1987; 80: 492–4
Bertschinger P, Himmelmann A, Risti B, et al. Cyclosporine treatment of severe ulcerative colitis during pregnancy [comment]. Am J Gastroenterol 1995; 90(2): 330
Flechner SM, Katz AR, Rogers AJ, et al. The presence of cyclosporine in body tissues and fluids during pregnancy. Am J Kidney Dis 1985; 5(1): 60–3
Thiru Y, Bateman DN, Coulthard MG. Successful breast feeding while mother was taking cyclosporin. BMJ 1997; 315: 463
Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. N Engl J Med 1995; 332: 292–7
Subhani JM, Hamilton MI. Review article: the management of inflammatory bowel disease during pregnancy. Aliment Pharmacol Ther 1998; 12: 1039–53
Milunsky A, Graef JW, Gaynor MF. Methotrexate-induced congenital malformations. J Pediatr 1968; 72: 790–5
Powell HR, Ekert H. Methotrexate-induced congenital malformations. Med J Aust 1971; 2: 1076–7
Feldkamp M, Carey JC. Clinical teratology counselling and consultation case report: low dose methotrexate exposure in the early weeks of pregnancy. Teratology 1993; 47(6): 533–9
Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first-trimester exposure to low-dose methotrexate in eight patients with rheumatic disease. Am J Med 1990; 88(6): 589–92
Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low-dose methotrexate treatment of the mother. Arthritis Rheum 1997; 40(5); 971–3
Nicholson HO. Cytotoxic drugs in pregnancy: a review of reported cases. J Obstet Gynaecol 1968; 75: 307–12
Pizzuto J, Aviles A, Noriega L, et al. Treatment of acute leukemia during pregnancy: presentation of nine cases. Cancer Treat Rep 1980; 64: 79–83
Schleuning M, Clemm C. Chromosomal aberrations in a newborn whose mother received cytotoxic treatment during pregnancy. N Engl J Med 1987; 317: 1666–7
Johns DG, Rutherford LD, Leighton PC, et al. Secretion of methotrexate into human milk. Am J Obstet Gynecol 1972; 112: 978–80
Ursing B, Alm T, Barany F, et al. A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: the cooperative Crohn’s disease study in Sweden: II: results. Gastroenterology 1982; 83(3): 550–62
Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole for the prevention of Crohn’s recurrence after ileal resection. Gastroenterology 1995; 108: 1617–21
Dashe JS, Gilstrap LC. Antibiotic use in pregnancy. Obstet Gynecol Clin North Am 1997; 24(3): 617–29
Heisterberg L. Placental transfer of metronidazole in the first trimester of pregnancy. J Perinatol Med 1984; 12: 43–5
Burtin P, Taddio A, Ariburnu O, et al. Safety of metronidazole in pregnancy: a meta-analysis. Am J Obstet Gynecol 1995; 172: 525–9
Caro-Paton T, Carvajal A, de Diego IM, et al. Is metronidazole teratogenic?: a meta-analysis. Br J Clin Pharmacol 1997; 44(2): 179–82
Dobias L, Cerna M, Rossner P, et al. Genotoxicity and carcinogenicity of metronidazole. Mutat Res 1994; 317: 177–94
Erikson SH, Oppenheim GL, Smith GH. Metronidazole in breast milk. Obstet Gynecol 1981; 57(1): 48–50
Clements CJ. Metronidazole and breast feeding. N Z Med J 1980; 92: 329
Berkovitch M, Pastuszak A, Gazarian M, et al. Safety of the new quinolones in pregnancy. Obstet Gynecol 1994; 84(4): 535–8
Mayer DG. Overview of toxicological studies. Drugs 1987; 34 Suppl. 1: 150–3
Schluter G. Ciprofloxacin: toxicologic evaluation of additional safety data. Am J Med 1989; 87 Suppl. 5A: 37–9
Giamarellou H, Kolokythas E, Petrikkos G, et al. Pharmacokinetics of three newer quinolones in pregnant and lactating women. Am J Med 1989; 87(5A): 49S–51S
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Connell, W., Miller, A. Treating Inflammatory Bowel Disease During Pregnancy. Drug-Safety 21, 311–323 (1999). https://doi.org/10.2165/00002018-199921040-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-199921040-00006