Abstract
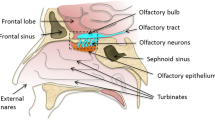
The connection between the nasal cavity and the CNS by the olfactory neurones has been investigated extensively during the last decades with regard to its feasibility to serve as a direct drug transport route to the CSF and brain. This drug transport route has gained much interest as it may circumvent the blood-brain barrier (BBB), which prevents some drugs from entering the brain. Approximately 100 published papers mainly reporting animal experiments were reviewed to evaluate whether the experimental design used and the results generated provided adequate pharmacokinetic information to assess whether the investigated drug was transported directly from the olfactory area to the CNS. In the analysis the large anatomical differences between the olfactory areas of animals and humans and the experimental conditions used were evaluated. The aim of this paper was to establish the actual evidence for the feasibility of this direct transport route in humans.
Twelve papers presented a sound experimental design to study direct nose to CNS transport of drugs based on the authors’ criteria. Of these, only two studies in rats were able to provide results that can be seen as an indication for direct transport from the nose to the CNS. No pharmacokinetic evidence could be found to support a claim that nasal administration of drugs in humans will result in an enhanced delivery to their target sites in the brain compared with intravenous administration of the same drug under similar dosage conditions.





Similar content being viewed by others
Notes
The total number of claims (n =17)exceeds the number of papers (n =12)as some papers tested more than one compound.
References
Pardridge WM. Drug delivery to the brain. J Cereb Blood Metab 1997; 17 (7): 713–31
Barnett EM, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology 1993; (1): 185–91
Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chemical Senses 2001; 26 (4): 433–45
Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J Comp Neurol 1990; 297 (1): 1–13
Anand Kumar TC, David GFX, Umberkoman B, et al. Uptake of radioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled oestradiol and progesterone. Curr Sci 1974; 43 (14): 435–9
Anand Kumar TC, David GFX, Puri V. Nasal spray and contraceptives. In: Talwar GP, editor. Recent advances in reproduction and regulation of fertility. Amsterdam: Elsevier/North-Holland Biomedical Press, 1979: 49–56
Anand Kumar TC, David GFX, Sankaranarayanan A, et al. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc Natl Acad Sci U S A 1982; 79 (13): 4185–9
David GFX, Puri CP, Anand Kumar TC. Bioavailability of progesterone enhanced by intranasal spraying. Experientia 1981; 37 (5): 533–4
Dluzen DE, Kefalas G. The effects of intranasal infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) upon catecholamine concentrations within olfactory bulbs and corpus striatum of male mice. Brain Res 1996; 741 (1–2): 215–9
Shimizu H, Oh IS, Okada S, et al. Inhibition of appetite by nasal leptin administration in rats. Int J Obes 2005; 29 (7): 858–63
Gozes I, Bardea A, Reshef A, et al. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci U S A 1996; 93 (1): 427–32
Frey WH, Liu J, Chen X, et al. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv 1997; 4 (2): 87–92
Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004; 29 (10): 1326–34
Born J, Lange T, Kern W, et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002; 5 (6): 514–6
Capsoni S, Giannotta S, Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Natl Acad Sci U S A 2002; 99 (19): 12432–7
Chen XQ, Fawcett JR, Rahman YE, et al. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis 1998; 1 (1): 35–44
Denecke H, Czehak N, Pietrowsky R. Dose-response relationships of intranasal cholecystokinin and the P300 event-related brain potential. Pharmacol Biochem Behav 2002; 73 (3): 593–600
Denecke H, Meyer F, Feldkamp J, et al. Repetitive intranasal administration of cholecystokinin potentiates its central nervous effects. Physiol Behav 2004; 83 (1): 39–45
De Rosa R, Garcia AA, Braschi C, et al. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A 2005; 102 (10): 3811–6
Frey WH, Liu J, Thorne RG, et al. Intranasal delivery of I-125-labeled nerve growth factor to the brain via the olfactory route. In: Iqbal K, Mortimer JA, Winblad B, et al., editors. Research advances in Alzheimer’s disease and related disorders. Chichester: John Wiley & Son Ltd, 1995: 329–35
Hallschmid M, Benedict C, Schultes B, et al. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004; 53 (11): 3024–9
Jin KL, Xie L, Childs J, et al. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann Neurol 2003; 53 (3): 405–9
Kern W, Born J, Schreiber H, et al. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 1999; 48 (3): 557–63
Krude H, Biebermann H, Schnabel D, et al. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab 2003; 88 (10): 4633–40
Kumbale R, Frey WH, Wilson S, et al. GM1 delivery to the CSF via the olfactory pathway. Drug Deliv 1999; 6 (1): 23–30
Liu XF, Fawcett JR, Hanson LR, et al. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis 2004; 13 (1): 16–23
Perras B, Marshall L, Köhler G, et al. Sleep and endocrine changes after intranasal administration of growth hormonereleasing hormone in young and aged humans. Psychoneuroendocrinology 1999; 24 (7): 743–57
Perras B, Wagner U, Born J, et al. Improvement of sleep and pituitary-adrenal inhibition after subchronic intranasal vasopressin treatment in elderly humans. J Clin Psychopharmacol 2003; 23 (1): 35–44
Smolnik R, Molle M, Fehm HL, et al. Brain potentials and attention after acute and subchronic intranasal administration of ACTH 4-10 and desacetyl-alpha-MSH in humans. Neuroendocrinology 1999; 70 (1): 63–72
Thorne RG, Pronk GJ, Padmanabhan V, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004; 127 (2): 481–96
Wang Y, Aun R, Tse FL. Brain uptake of dihydroergotamine after intravenous and nasal administration in the rat. Biopharm Drug Dispos 1998; 19 (9): 571–5
Zhao HM, Liu XF, Mao XW, et al. Intranasal delivery of nerve growth factor to protect the central nervous system against acute cerebral infarction. Chin Med Sci J 2004; 19 (4): 257–61
Chow HHS, Anavy N, Villalobos A. Direct nose-brain transport of benzoylecgonine following intranasal administration in rats. J Pharm Sci 2001; 90 (11): 1729–35
Dufes C, Olivier JC, Gaillard F, et al. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int J Pharm 2003; 255 (1–2): 87–97
Kao HD, Traboulsi A, Itoh S, et al. Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. Pharm Res 2000; 17 (8): 978–84
Sakane T, Akizuki M, Yamashita S, et al. The transport of a drug to the cerebrospinal fluid directly from the nasal cavity: the relation to the lipophilicity of the drug. Chem Pharm Bull 1991; 39 (9): 2456–8
Sakane T, Akizuki M, Yamashita S, et al. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol 1994; 46 (5): 378–9
Sakane T, Akizuki M, Taki Y, et al. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol 1995; 47 (5): 379–81
Sakane T, Yamashita S, Yata N, et al. Transnasal delivery of 5-fluorouracil to the brain in the rat. J Drug Target 1999; 7 (3): 233–40
Seki T, Sato N, Hasegawa T, et al. Nasal absorption of zidovudine and its transport to cerebrospinal fluid in rats. Biol Pharm Bull 1994; 17 (8): 1135–7
Hussain A, Hirai S, Bawarshi R. Nasal absorption of propranolol from different dosage forms by rats and dogs. J Pharm Sci 1980; 69 (12): 1411–3
Hirai S, Yahiki T, Tai M, et al. Absorption of drugs from the nasal mucosa of rat. Int J Pharm 1981; 7 (4): 317–25
Van den Berg MP, Merkus P, Romeijn SG, et al. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J Drug Target 2003; 11 (6): 325–31
Van den Berg MP, Merkus P, Romeijn SG, et al. Uptake of melatonin into the cerebrospinal fluid after nasal and intravenous delivery: studies in rats and comparison with a human study. Pharm Res 2004; 21 (5): 799–802
Merkus P, Guchelaar HJ, Bosch DA, et al. Direct access of drugs to the human brain after intranasal drug administration? Neurology 2003; 60 (2): 1669–71
Wall A, K/0agedal M, Bergström M, et al. Distribution of zolmitriptan into the CNS in healthy volunteers: a positron emission tomography study. Drugs R D 2005; 6 (3): 139–47
Chandler SG, Illum L, Thomas NW. Nasal absorption in rat. I: A method to demonstrate the histological effects of nasal formulations. Int J Pharm 1991; 70 (1–2): 19–27
Hunt CA, MacGregor RD, Siegel RA. Engineering targeted in vivo drug delivery. I: The physiological and physiochemical principles governing opportunities and limitations. Pharm Res 1986; 3 (6): 333–44
Chow HS, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci 1999; 88 (8): 754–8
Hussain MA, Rakestraw D, Rowe S, et al. Nasal administration of a cognition enhancer provides improved bioavailability but not enhanced brain delivery. J Pharm Sci 1990; 79 (9): 771–2
Shi J, Perry G, Berridge MS, et al. Labeling of cerebral amyloid beta deposits in vivo using intranasal basic fibroblast growth factor and serum amyloid P component in mice. J Nucl Med 2002; 43 (8): 1044–51
Dahlin M, Bergman U, Jansson B, et al. Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharm Res 2000; 17 (6): 737–42
Char H, Kumar S, Patel S, et al. Nasal delivery of [14C]dextromethorphan hydrochloride in rats: levels in plasma and brain. J Pharm Sci 1992; 81 (8): 750–2
Vyas TK, Babbar AK, Sharma RK, et al. Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target 2005; 13 (5): 317–24
Bergström U, Franzén A, Eriksson C, et al. Drug targeting to the brain: transfer of picolinic acid along the olfactory pathways. J Drug Target 2002; 10 (6): 469–78
Eriksson C, Bergman U, Franzén A, et al. Transfer of some carboxylic acids in the olfactory system following intranasal administration. J Drug Target 1999; 7 (2): 131–42
Ross TM, Martinez PM, Renner JC, et al. Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol 2004; 151 (1–2): 66–77
Wang H, Hussain AA, Wedlund PJ. Nipecotic acid: systemic availability and brain delivery after nasal administration of nipecotic acid and n-butyl nipecotate to rats. Pharm Res 2005; 22 (4): 556–62
Westin U, Piras E, Jansson B, et al. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci 2005; 24 (5): 565–73
Sigurdsson P, Thorvaldsson T, Gizurarson S. Olfactory absorption of insulin to the brain. Drug Deliv 1997; 4 (3): 195–200
Khopade AJ, Mahadik KR, Jain NK. Enhanced brain uptake of rifampicin from W/O/W multiple emulsions via nasal route. Indian J Pharm Sci 1996; 58 (2): 83–5
Jansson B, Hägerström H, Fransén N, et al. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur J Pharm Biopharm 2005; 59 (3): 557–64
Jansson B, Björk E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J Drug Target 2002; 10 (5): 379–86
Larsson P, Tjälve H. Intranasal instillation of aflatoxin B1 in rats: bioactivation in the nasal mucosa and neuronal transport to the olfactory bulb. Toxicol Sci 2000; 55 (2): 383–91
Lindquist NG, Lydén A, Narfström K, et al. Accumulation of taurine in the nasal mucosa and the olfactory bulb. Experientia 1983; 39 (7): 797–9
Persson E, Larsson P, Tjälve H. Cellular activation and neuronal transport of intranasally instilled benzo(a)pyrene in the olfactory system of rats. Toxicol Lett 2002; 133 (2s-3): 211–9
Westin UE, Boström E, Gr/0asjö J, et al. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res 2006; 23 (3): 565–72
Gizurarson S, Thorvaldsson T, Sigurdsson P, et al. Selective delivery of insulin into the brain: intraolfactory absorption. Int J Pharm 1997; 146 (1): 135–41
Anand Kumar TC, David GFX, Kumar K, et al. A new approach to fertility regulation by interfering with neuroendocrine pathways. In: Anand Kumar TC, editor. Neuroendocrine regulation of fertility. Basel: Karger, 1976: 314–22
Sakane T, Akizuki M, Yoshida M, et al. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol 1991; 43 (6): 449–51
Chou KJ, Donovan MD. Distribution of antihistamines into the CSF following intranasal delivery. Biopharm Drug Dispos 1997; 18 (4): 335–46
Chou KJ, Donovan MD. Lidocaine distribution into the CNS following nasal and arterial delivery: a comparison of local sampling and microdialysis techniques. Int J Pharm 1998; 171 (1): 53–61
Chou KJ, Donovan MD. The distribution of local anesthetics into the CSF following intranasal administration. Int J Pharm 1998; 168 (2): 137–45
Henry RJ, Ruano N, Casto D, et al. A pharmacokinetic study of midazolam in dogs: nasal drops vs. atomizer administration. Pediatr Dent 1998; 20 (5): 321–6
Al-Ghananeem AM, Traboulsi AA, Dittert LW, et al. Targeted brain delivery of 17beta-estradiol via nasally administered water soluble prodrugs. AAPS PharmSciTech 2002; 3 (1): 8
Dahlin M, Björk E. Nasal administration of a physostigmine analogue (NXX-066) for Alzheimer’s disease to rats. Int J Pharm 2001; 212 (2): 267–74
Dahlin M, Björk E. Nasal absorption of (S)-UH-301 and its transport into the cerebrospinal fluid of rats. Int J Pharm 2000; 195 (1–2): 197–205
Yajima T, Juni K, Saneyoshi M, et al. Direct transport of 2’,3’-didehydro-3’-deoxythymidine (D4T) and its ester derivatives to the cerebrospinal fluid via the nasal mucous membrane in rats. Biol Pharm Bull 1998; 21 (3): 272–7
Yang Z, Huang Y, Gan G, et al. Microdialysis evaluation of the brain distribution of stavudine following intranasal and intravenous administration to rats. J Pharm Sci 2005; 94 (7): 1577–88
Bagger M, Bechgaard E. A microdialysis model to examine nasal drug delivery and olfactory absorption in rats using lidocaine hydrochloride as a model drug. Int J Pharm 2004; 269 (2): 311–22
Bagger MA, Bechgaard E. The potential of nasal application for delivery to the central brain: a microdialysis study for fluorescein in rats. Eur J Pharm Sci 2004; 21 (2–3): 235–42
Wang F, Jiang X, Lu W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int J Pharm 2003; 263 (1–2): 1–7
Van den Berg MP, Verhoef JC, Romeijn SG, et al. Uptake of hydrocortisone into the cerebrospinal fluid of rats: comparison of intranasal and intravenous administration in the same animal. STP Pharma Sci 2002; 12 (4): 251–5
Van den Berg MP, Verhoef JC, Romeijn SG, et al. Uptake of estradiol or progesterone into the CSF following intranasal and intravenous delivery in rats. Eur J Pharm Biopharm 2004; 58 (1): 131–5
In ’t Veen JPM, Van den Berg MP, Romeijn SG, et al. Uptake of fluorescein isothiocyanate-labelled dextran into the CSF after intranasal and intravenous administration to rats. Eur J Pharm Biopharm 2005; 61 (1–2): 27–31
Ang VTY, Jenkins JS. Blood-cerebrospinal fluid barrier to arginine-vasopressin, desmopressin and desglycinamide arginine-vasopressin in the dog. J Endocrinol 1982; 93 (3): 319–25
Madrid MY, Langer R. Intranasal drug delivery to the central nervous system. Proceedings of the International Symposium of the Controlled Release of Bioactive Materials. Amsterdam, The Netherlands, 1991: 4
Öhman L, Hahnenberger R, Johansson EDB. 17?-Estradiol levels in blood and cerebrospinal fluid after ocular and nasal administration in women and female rhesus monkeys (Macaca mulatta). Contraception 1980; 22 (4): 349–58
Dahlin M, Jansson B, Björk E. Levels of dopamine in blood and brain following nasal administration to rats. Eur J Pharm Sci 2001; 14 (1): 75–
Shi ZQ, Zhang QH, Jiang XG. Pharmacokinetic behavior in plasma, cerebrospinal fluid and cerebral cortex after intranasal administration of hydrochloride meptazinol. Life Sci 2005; 77 (20): 2574–83
Zhang Q, Jiang X, Jiang W, et al. Preparation of nimodipineloaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm 2004; 275 (1–2): 85–96
Lerner EN, Van Zanten EH, Stewart GR. Enhanced delivery of octreotide to the brain via transnasal iontophoretic administration. J Drug Target 2004; 12 (5): 273–80
Banks WA, During MJ, Niehoff ML. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9-39) after intranasal administration. J Pharmacol Exp Ther 2004; 309 (2): 469–75
Burstein AH, Modica R, Hatton M, et al. Pharmacokinetics and pharmacodynamics of midazolam after intranasal administration. J Clin Pharmacol 1997; 37 (8): 711–8
Gais S, Sommer M, Fischer S, et al. Post-trial administration of vasopressin in humans does not enhance memory formation (vasopressin and memory consolidation). Peptides 2002; 23 (3): 581–3
Gozes I, Giladi E, Pinhasov A, et al. Activity-dependent neurotrophic factor: intranasal administration of femtomolar-acting peptides improves performance in a water maze. J Pharmacol Exp Ther 2000; 293 (3): 1091–8
Hruz P, Zechner S, Heimberg D, et al. Intranasal administration of delta sleep-inducing peptide increases P300. J Clin Psychopharmacol 2001; 21 (6): 626–8
Lindhardt K, Gizurarson S, Stefánsson SB, et al. Electroencephalographic effects and serum concentrations after intranasal and intravenous administration of diazepam to healthy volunteers. Br J Clin Pharmacol 2001; 52 (5): 521–752
Reger MA, Watson GS, Frey II WH, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 2006; 27 (3): 451–8
Volkow ND, Wang GJ, Fischman MW, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 2000; 67 (12): 1507–15
Derad I, Willeke K, Pietrowsky R, et al. Intranasal angiotensin II directly influences central nervous regulation of blood pressure. Am J Hypertens 1998; 11 (8): 971–7
Pietrowsky R, Struben C, Molle M, et al. Brain potential changes after intranasal vs intravenous administration of vasopressin: evidence for a direct nose brain pathway for peptide effects in humans. Biol Psychiatry 1996; 39 (5): 332–40
Pietrowsky R, Thiemann A, Kern W, et al. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology 1996; 21 (6): 559–72
Fehm HL, Smolnik R, Kern W, et al. The melanocortin melanocyte-stimulating hormone/adrenocorticotropin4-10 decreases body fat in humans. J Clin Endocrinol Metab 2001; 86 (3): 1144–8
De Souza Silva MA, Mattern C, Hacker R, et al. Intranasal administration of the dopaminergic agonists L-DOPA, amphetamine, and cocaine increases dopamine activity in the neostriatum: a microdialysis study in the rat. J Neurochem 1997; 68 (1): 233–9
Schulz C, Paulus K, Lehnert H. Central nervous and metabolic effects of intranasally applied leptin. Endocrinology 2004; 145 (6): 2696–701
Pelidou SH, Zou LP, Deretzi G, et al. Intranasal administration of recombinant mouse interleukin-12 increases inflammation and demyelination in chronic experimental autoimmune neuritis in Lewis rats. Scand J Immunol 2000; 51 (1): 29–35
Yura M, Takahashi I, Terawaki S, et al. Nasal administration of cholera toxin (CT) suppresses clinical signs of experimental autoimmune encephalomyelitis (EAE). Vaccine 2001; 20 (1–2): 134–9
Pietrowsky R, Claassen L, Frercks H, et al. Time course of intranasally administered cholecystokinin-8 on central nervous effects. Neuropsychobiology 2001; 43 (4): 254–9
Weiner HL, Lemere CA, Maron R, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol 2000; 48 (4): 567–79
Yu YP, Xu QQ, Zhang Q, et al. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett 2005; 387 (1): 5–10
Liu XF, Fawcett JR, Thorne RG, et al. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci 2001; 187 (1–2): 91–7
Liu XF, Fawcett JR, Thorne RG, et al. Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett 2001; 308 (2): 91–4
Zhang QZ, Jiang XG, Wu CH. Distribution of nimodipine in brain following intranasal administration in rats. Acta Pharmacol Sin 2004; 25 (4): 522–7
Van den Berg MP, Romeijn SG, Verhoef JC, et al. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J Neurosci Methods 2002; 116 (1): 99–107
Chou RC, Levy G. Effect of heparin or salicylate infusion on serum protein binding and on concentrations of phenytoin in serum, brain and cerebrospinal fluid of rats. J Pharmacol Exp Ther 1981; 219 (1): 42–8
Waynforth, HB, ai]Flecknell, PA. Cisternal puncture (and intracisternal injection). In: Waynforth HB, Flecknell PA, editors. Experimental and surgical techniques in the rat. London: Academic Press, 1980: 59–61
Acknowledgements
The authors used no sources of funding in the writing of this article. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merkus, F.W.H.M., van den Berg, M.P. Can Nasal Drug Delivery Bypass the Blood-Brain Barrier?. Drugs R D 8, 133–144 (2007). https://doi.org/10.2165/00126839-200708030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200708030-00001