Abstract
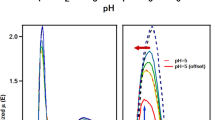
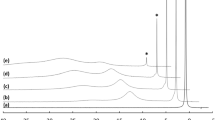
Ligand exchange reactions of a monomeric zirconium carbonate complex with carboxylic acids were studied by means of extended X-ray absorption fine structure (EXAFS), UV absorption spectrophotometry and Raman spectrometry. Three carboxylic acids, gluconic acid, and L-tartaric acid and citric acid, which are mono-, di- and tri-carboxylic acids, respectively, were employed in this study. These three carboxylic acids gave different spectral signatures and concentration dependences, respectively. In the gluconic acid system, the peaks on Fourier transform of EXAFS spectrum and Raman spectrum caused by carbonate ion coordinating to zirconium atom were obviously decreased with increasing gluconic acid concentration compared to the other two carboxylic acid systems. This indicates the high association ability of gluconic acid to zirconium, which was revealed by UV spectrophotometric analysis.
Similar content being viewed by others
References
I. Denry and J. R. Kelly, Dent. Mater., 2008, 24, 299.
C. Piconi and G. Maccauro, Biomaterials, 1999, 20, 1.
M. P. Staiger, A. M. Pietak, J. Huadmai, and G. Dias, Biomaterials, 2006, 27, 1728.
D. W. Shoesmith and D. Zagidulin, J. Nucl. Mater., 2011, 418, 292.
W. Jo, R. Dittmer, M. Acosta, J. Zang, C. Groh, E. Sapper, K. Wang, and J. Rödel, J. Electroceram., 2012, 29, 71.
A. J. Gaunt, I. May, D. Collison, K. Travis Holman, and M. T. Pope, J. Mol. Struct., 2003, 656, 101.
M. Sanz, M. E. G. Mosquera, and T. Cuenca, Dalton Trans., 2009, 2616.
A. Clearfield and Z. Wang, J. Chem. Soc. Dalton Trans., 2002, 2937.
K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034.
E. Farnworth, S. L. Jones, and I. McAlpine, “The Production, Properties, and Uses of Zirconium Chemicals”, in “Specialty Inorganic Chemicals”, ed. R. Thompson, 1980, Royal Society of Chemistry, London, 248.
C. Walther, J. Rothe, M. Fuss, S. Büchner, S. Koltsov, and T. Bergmann, Anal. Bioanal. Chem., 2007, 388, 409.
P. A. Lee, P. H. Citrin, P. Eisenberger, and B. M. Kincaid, Reviews of Modern Physics, 1981, 53, 769.
F. Takasaki, K. Fujiwara, Y. Nakajima, T. Nishikawa, and N. Ogawa, Chem. Lett., 2014, 43, 196.
F. Takasaki, K. Fujiwara, Y. Nakajima, T. Nishikawa, H. Masu, M. Imanari, Y. Hidaka, and N. Ogawa, Dalton Trans., 2015, 44, 645.
F. Takasaki, N. Ogawa, I. Watanabe, T. Suzuki, Y. Nakajima, T. Wakita, and R. Suzuki, Bunseki Kagaku, 2010, 59, 447.
P. L. Brown, E. Curti, B. Grambow, and C. Ekberg, “Chemical Thermodynamics of Zirconium”, ed. F. J. Mompean, J. Perrone, and M. Illemassène, 2006, Elsevier Science.
M. T. Beck and I. Nagypal, “Chemistry of Complex Equilibria, Enlarged and Completely Revised Version”, ed. E. Horwood, 1990, Halsted Press, Australia.
D. K. Chakravorty, R. Ghosh, R. Banerjee, and D. Sarkar, Polyhedron, 2009, 28, 1315.
For example, commercially available as “Ammonium Zirconium(IV) Carbonate Solution in H2O, Contains 1-2% Tartaric Acid as Stabilizer”, from Sigma-Aldrich, CAS No. 12616-24-9.
E. M. Larsen and E. H. Homeier, Inorg. Chem., 1972, 11, 2687.
A. Clearfield, Inorg. Chim. Acta, 1970, 4, 166.
S. I. Zabinsky, J. J. Rehr, A. Ankudinov, R. C. Albers, and M. J. Eller, Phys. Rev. B, 1995, 52, 2995.
C. V. Kumar and Z. J. Williams, J. Phys. Chem., 1995, 99, 17632.
F. Takasaki, Japan Patent, 2017, 6103818.
Acknowledgments
The synchrotron radiation EXAFS experiment was performed at the BL14B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2010B1961, 2011B1826 and 2012A1777). We would like to thank Prof. Iwao Watanabe, Dr. Hiroshi Oji and Dr. Yosuke Taniguchi for help on EXAFS measurements and analyses.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Takasaki, F., Fujiwara, K., Kikuchi, T. et al. Ligand Exchange Reactions of a Monomeric Zirconium Carbonate Complex with Carboxylic Acids Studied by Extended X-ray Absorption Fine Structure, UV Absorption and Raman Spectrophotometry. ANAL. SCI. 33, 1007–1012 (2017). https://doi.org/10.2116/analsci.33.1007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.33.1007