Abstract
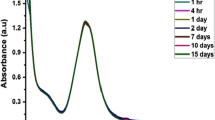
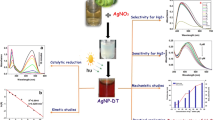
In the current work a uniform morphological Ag nanoparticles (Ag NPs) were prepared with ascorbic acid as a reducing agent and citrate as a stabilizer. The surface of Ag NPs modified by crystal violet (CV) and potassium iodide (KI) was used as an aggregation agent to obtain CV modified Ag NPs (CV-Ag NPs) probes for detecting mercury ions. The mercury ions could be reduced to mercury molecules by citrate, and then deposited on the surface of Ag NPs, leading to the separation of CV molecules from the surface of Ag NPs. Therefore, the SERS signal intensity of CV decreased with the increase of the Hg2+ concentration and the concentration of Hg2+ was in the range of 1 × 10–11 to 1 × 10–5 M. Taking the change of the characteristic peak intensity of CV at 913 cm–1 as a reference, the SERS spectrum intensity of CV has a linear relationship with the Hg2+ concentration. The equation is y =–333.55x + 1343.05, where the linear correlation coefficient is R2 = 0.980, and the recovery rate is between 84.20 to 105.60%. Finally, the CV-Ag NPs probe was used to quickly detect soluble mercury in cinnabar. Compared with the conventional large-scale instrument detection method, this simple and fast method, can be applied for rapid detection of soluble mercury, and has a certain significance for concerning the research of mineral medicine processing mechanism.
Similar content being viewed by others
References
X. Zhou, L. Wang, X. Sun, X. Yang, C. Chen, Q. Wang, and X. Yang, J. Ethnopharmacol., 2011, 135, 110.
S. Cao, J. Xia, L. Li, H. Chen, X. Yang, and S. Ji, Lishizhen Med. Mater. Med. Res., 2016, 27, 1110.
X. Zhou, K. Zeng, Q. Wang, X. Yang, and K. Wang, J. Ethnopharmacol., 2010, 131, 196.
P. Jiang, Y. Li, G. Liu, G. Yang, L. Lagos, Y. Yin, B. Gu, G. Jiang, and Y. Cai, J. Hazard. Mater., 2016, 317, 466.
J. S. Waples, K. L. Nagy, G. R. Aiken, and J. N. Ryan, Geochim. Cosmochim. Acta, 2005, 69, 1575.
R. J. Huang, Z. X. Zhuang, Y. Tai, R. F. Huang, X. R. Wang, and F. S. C. Lee, Talanta, 2006, 68, 728.
Q. T. Jiang, L. Zeng, J. Ma, L. N. Peng, W. L. Li, Y. Ding, and J. Li, Chin. J. Anal. Chem., 2016, 44, 979.
Q. Wu, X. He, S. J. Zhou, F. G. Shi, and Y. F. Lu, Toxicol. In Vitro, 2020, 63, 7.
J. Langer, D. Jimenez De Aberasturi, J. Aizpurua, R. A. Alvarez-Puebla, B. Auguie, J. J. Baumberg, G. C. Bazan, S. E. J. Bell, A. Boisen, A. G. Brolo, J. Choo, D. Cialla-May, V. Deckert, L. Fabris, K. Faulds, F. J. Garcia De Abajo, R. Goodacre, D. Graham, A. J. Haes, C. L. Haynes, C. Huck, T. Itoh, M. Kall, J. Kneipp, N. A. Kotov, H. Kuang, E. C. Le Ru, H. K. Lee, J. F. Li, X. Y. Ling, S. A. Maier, T. Mayerhofer, M. Moskovits, K. Murakoshi, J. M. Nam, S. Nie, Y. Ozaki, I. Pastoriza-Santos, J. Perez-Juste, J. Popp, A. Pucci, S. Reich, B. Ren, G. C. Schatz, T. Shegai, S. Schlucker, L. L. Tay, K. G. Thomas, Z. Q. Tian, R. P. Van Duyne, T. Vo-Dinh, Y. Wang, K. A. Willets, C. Xu, H. Xu, Y. Xu, Y. S. Yamamoto, B. Zhao, and L. M. Liz-Marzan, ACS Nano, 2020, 14, 28.
A. B. Zrimsek, N. Chiang, M. Mattei, S. Zaleski, M. O. Mcanally, C. T. Chapman, A. I. Henry, G. C. Schatz, and R. P. Van Duyne, Chem. Rev., 2017, 117, 7583.
N. Li, S. Han, C. Zhang, S. Lin, X. Y. Sha, and W. Hasi, Anal. Sci., 2020, 36, 935.
S. Lin, X. Lin, S. Han, H. Zhao, W. Hasi, and L. Wang, Nanotechnology, 2019, 30, 215601.
X. Lin, G. Fang, Y. Liu, Y. He, L. Wang, and B. Dong, J. Phys. Chem. Lett., 2020, 11, 3573.
S. Y. Fu, X. Y. Guo, H. Wang, T. X. Yang, Y. Wen, and H. F. Yang, Sens. Actuators, B, 2014, 199, 108.
W. Ren, C. Zhu, and E. Wang, Nanoscale, 2012, 4, 5902.
Y. Qi, J. Zhao, G.-J. Weng, J.-J. Li, X. Li, J. Zhu, and J.-W. Zhao, J. Mater. Chem. C, 2018, 6, 12283.
K. Li, A. Liang, C. Jiang, F. Li, Q. Liu, and Z. Jiang, Talanta, 2012, 99, 890.
I. Ojea-Jimenez, X. Lopez, J. Arbiol, and V. Puntes, ACS Nano, 2012, 6, 2253.
Y. Q. Qin, X. H. Ji, J. Jing, H. Liu, H. L. Wu, and W. S. Yang, Colloids Surf. A, 2010, 372, 172.
P. Pinkhasova, H. Chen, M. W. G. M. Verhoeven, S. Sukhishvili, and H. Du, RSC Adv., 2013, 3, 17954.
S. Lin, X. Lin, S. Q. G. W. Han, L. He, H. Y. Zhao, J. Zhang, W. L. J. Hasi, and L. Wang, J. Alloys Compd., 2019, 805, 318.
L. He, N. J. Kim, H. Li, Z. Hu, and M. Lin, J. Agric. Food Chem., 2008, 56, 9843.
S. Q. Han, X. Chen, C. Zhang, H. Zhao, S. Lin, Y. Zhang, and W. L. Hasi, Anal. Sci., 2019, 35, 1209.
C. Zhang, S. Q. Han, H. Zhao, S. Lin, and W. L. Hasi, Anal. Sci., 2018, 34, 1249.
L. Xie, J. Lu, T. Liu, G. Chen, G. Liu, B. Ren, and Z. Tian, J. Phys. Chem. Lett., 2020, 11, 1022.
C. Zhu, G. Meng, P. Zheng, Q. Huang, Z. Li, X. Hu, X. Wang, Z. Huang, F. Li, and N. Wu, Adv. Mater., 2016, 28, 4871.
T. Kulikova, E. Hiller, L. Jurkovic, L. Filova, P. Sottnik, and P. Lacina, Environ. Monit. Assess., 2019, 191, 263.
R. L. Frost, S. Bahfenne, and E. C. Keeffe, J. Raman Spectrosc., 2010, 41, 1779.
X. Yuan, K. Li, Y. Zhang, Y. Miao, Y. Xiang, Y. Sha, M. Zhang, and K. Huang, Microchem. J., 2020, 155, 1.
L. F. Shi, D. F. Xue, H. G. Xu, H. Liu, and W. F. Teng, Spectrosc. Spectr. Anal., 2007, 27, 1036.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31871873 and 82160806).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, N., Han, S., Lin, S. et al. Detection of Soluble Mercury in Cinnabar Using a CV-Ag NPs SERS Probe. ANAL. SCI. 37, 1407–1412 (2021). https://doi.org/10.2116/analsci.21P047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.21P047