Abstract
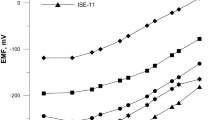
New polymeric membrane (PME) and coated graphite (CGE) copper(II)-selective electrodes based on 1-hydroxy-2-(prop-2′-enyl)-4-(prop-2′-enyloxy)-9,10-anthraquinone were prepared. The electrodes reveal linear emf-pCu2+ responses over wide concentration ranges (1.0 × 10–5–1.0 × 10–1 M with a slope of 27.3 mV decade–1 for PME and 8.0 × 10–8–5.0 × 10–2 M with a slope of 29.1 mV decade–1 for CGE) and very low limits of detection (8.0 × 10–6 M for PME and 5.0 × 10–8 M for CGE). The potentiometric response is independent of the pH of the test solution in the pH range 3.0–6.0. The proposed electrodes possess very good selectivities over a wide variety of other cations, including alkali, alkaline earth, transition and heavy metal ions, the selectivity coefficients for the CGE being much improved over those for the PME. The electrodes were used as indicator electrodes in the potentiometric titration of Cu2+ and in the recovery of copper ions from wastewater.
Similar content being viewed by others
References
M. E. Meyerhoff and M. N. Opdyche, Adv. Clin. Chem., 1986, 25, 1.
G. J. Moody, B. B. Saad, and J. D. R. Thomas, Sel. Electrode. Rev., 1988, 10, 71.
Y. Umezawa (ed.), “CRC Handbook of Ion-Selective Electrodes: Selectivity Coefficients”, 1990, CRC Press, Boca Raton.
J. Janata, M. Jasowicz, and D. M. DeVaney, Anal. Chem., 1994, 66, 207R.
P. Bühlmann, E. Pretsch, and E. Bakker, Chem. Rev., 1998, 98, 1593.
H. R. Marston, Physiol. Rev., 1952, 32, 56.
G. M. Morrison, in “Handbook on Metal-Ligand Interactions in Biological Fluids, ed. G. Berthon, 1995, Chap. 7, Dekker, New York.
B. Mason, “Principles of Geochemistry”, 1971, Wiley, New York.
N. M. Greenwood and A. Earnshaw, “Chemistry of Elements”, 1984, Pergamon Press, New York.
S. Kamata, Y. Yamasaki, M. Higo, A. Bhale, and Y. Fukunaga, Analyst, 1988, 113, 45.
S. Kamata, A. Bhale, Y. Fukunaga, and H. Murata, Anal. Chem., 1988, 60, 2464.
S. Kamata, Y. Kubo, H. Murata, and A. Bhale, Analyst, 1989, 114, 1029.
Z. Brzozka, Analyst, 1988, 113, 1803.
P. L. H. M. Cobben, R. J. M. Egberink, J. B. Bomer, P. Bergved, W. Verboom, and D. N. Reinhoudt, J. Am. Chem. Soc, 1992, 114, 10573.
M. Shamsipur, S. Rouhani, M. R. Ganjali, H. Eshghi, and H. Sharghi, Microchem. J., 1999, 63, 202.
K. Ren, Talanta, 1989, 113, 1803.
N. Alizadeh, S. Ershad, H. Naeimi, H. Sharghi, and M. Shamsipur, Fresenius J. Anal. Chem., 1999, 365, 511.
M. Frant, Analyst, 1994, 119, 2293.
D. A. Roman, Marine Chem., 1992, 38, 165.
S. Dadfarnia, M. Shamsipur, F. Tamaddon, and H. Sharghi, J. Memb. Sci., 1993, 78, 115.
F. Raoufi, Y. Yamini, H. Sharghi, and M. Shamsipur, Microchem. J., 1999, 63, 311.
M. Shamsipur, F. Raoufi, and H. Sharghi, Talanta, 2000, 52, 737.
N. Tavakkoli, Z. Khojasteh, H. Sharghi, and M. Shamsipur, Anal. Chim. Acta, 1998, 360, 203.
H. R. Pouretedal, A. Forghaniha, H. Sharghi, and M. Shamsipur, Anal. Lett., 1998, 31, 2591.
A. Rahmani, M. Barzegar, M. Shamsipur, H. Sharghi, and M. F. Mousavi, Anal. Lett., 2000, 33, 2611.
M. F. Mousavi, A. Rahmani, S. M. Golabi, M. Shamsipur, and H. Sharghi, Talanta, 2001, 55, 305.
H. Sharghi and A. Forghaniha, Iran. J. Chem. Chem. Eng., 1995, 14, 16.
M. G. Johnson, H. Kiyokawa, S. Tani, J. Koyama, S. L. Morris Natschke, A. Mauger, M. M. Bowers Daines, B. C. Lange, and K. H. Lee, Biorg. Med. Chem., 1997, 5, 1469.
M. Shamsipur, A. Avanes, G. Aghapour, and H. Sharghi, Polish J. Chem., 2001, 75, 1533.
X. Yang, N. Kumar, H. Chi, D. D. Hibbert, and P. N. W. Alexander, Electroanalysis, 1997, 9, 549.
E. Bakker, P. Bühlmann, and E. Pretsch, Chem. Rev., 1997, 97, 2083.
A. R. Fakhari, M. R. Ganjali, and M. Shamsipur, Anal. Chem., 1997, 69, 3693.
M. R. Ganjali, A. Moghimi, G. W. Buchanan, and M. Shamsipur, J. Inclusion Phenom., 1998, 30, 24.
M. R. Ganjali, A. Moghimi, and M. Shamsipur, Anal. Chem., 1998, 70, 5259.
M. Shamsipur, S. Rouhani, H. Sharghi, M. R. Ganjali, and H. Eshghi, Anal. Chem., 1999, 71, 4938.
M. Shamsipur, M. Yousefi, and M. R. Ganjali, Anal. Chem., 2000, 72, 2391.
IUPAC Analytical Chemistry Division. Commission on Analytical Nomenclature. Recommendations for Nomenclatureof Ion-Selective Electrodes, Pure Appl. Chem., 1976, 48, 127.
P. Schnierle, T. Kappes, and P. C. Hauser, Anal. Chem., 1998, 70, 3585.
M. K. Amini, S. Shahrokhian, and S. Tangestaninejad, Analyst, 1999, 124, 1319.
S. Shahrokhian, M. K. Amini, R. Kia, and S. Tangestaninejad, Anal. Chem., 2000, 72, 956.
M. Shamsipur, M. Yousefi, M. Hosseini, M. R. Ganjali, H. Sharghi, and H. Naeimi, Anal. Chem., 2001, 73, 2869.
Y. Umezawa, K. Umezawa, and H. Sato, Pure Appl. Chem., 1995, 67, 507.
K. Srinivasan and G. A. Rechnitz, Anal. Chem., 1969, 41, 1203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamsipur, M., Avanes, A., Javanbakht, M. et al. A 9,10-Anthraquinone Derivative Having Two Propenyl Arms as a Neutral Ionophore for Highly Selective and Sensitive Membrane Sensors for Copper(II) Ion. ANAL. SCI. 18, 875–879 (2002). https://doi.org/10.2116/analsci.18.875
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.18.875