
An analysis of macular ganglion cell complex in 7-year-old children in China: the Anyang Childhood Eye Study
Introduction
Glaucoma, an irreversible condition of the eye that can lead to blindness, is characterized by loss of retinal ganglion cells (RGCs), structural changes of the optic nerve head and retinal nerve fiber layer (RNFL), and visual field defects (1,2). The macular ganglion cell complex (GCC), comprising RNFL, ganglion cell layer (GCL), and inner plexiform layer (IPL), can improve the detection of glaucoma (3-5). GCC is superior to RNFL thickness evaluation for early investigation of damage caused by glaucoma, particularly in the diagnosis of very early glaucoma (6).
The gold standard clinical test for glaucoma diagnosis is standard automated perimetry (7), which shows visual field defects when 25% to 40% of RGCs are lost (8). However, this typically reliable and reproducible visual field test is often unsuccessful or difficult to interpret in children because it requires patient cooperation (9).
Optical coherence tomography (OCT) is a noninvasive structural diagnostic device that provides objective measurements and can reveal structural changes in the retina with high-resolution, cross-sectional images. Previous studies have shown that spectral-domain OCT (SD-OCT) can reproducibly measure RNFL thickness in children (10,11). However, the OCT normative databases only include data on adults over 18 and are limited by low representation of Asian subjects (12,13) and lack of child subjects (14). This limits the usefulness of OCT for children as it is inappropriate to compare their results with the adult database (8,9).
In this study, we used SD-OCT imaging to describe macular GCC thickness and its association with systematic and ocular parameters in a cohort of 7-year-old children in China. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tp-21-323).
Methods
Study design and population
A cross-sectional study was conducted involving 2,505 students from urban areas in Anyang, Henan Province, Central China. The study has been recognized elsewhere for its detailed methodology (15). The Anyang Childhood Eye Study (ACES) was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University, Beijing, China (No. TRECKY2018-030). Each student provided verbal consent and informed written consent was obtained from at least one parent.
Standardized examinations
The height and weight of the participants were measured using professional, automated, and integrated equipment (UAL6X, UOSIM Co., Ltd., Dalian, China).
All subjects underwent examination of visual acuity using LogMAR chart (Precision Vision, Woodstock, IL, USA) to assess distant vision and HOTV eye chart (Precision Vision) for near vision.
Optical biometry measurements were recorded and then cycloplegic autorefraction was performed 30 minutes after 2 drops of 1% cyclopentolate (Alcaine; Alcon, Fort Worth, TX, USA) and 1 drop of 0.5% tropicamide (Mydrin-P; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Refraction was defined as spherical equivalent (SE, sphere power + cylinder power/2) in diopters (D). Myopia was defined as SE <−0.5 D, hyperopia as SE >+0.5 D and emmetropia as −0.5 D ≤ SE ≤ +0.5 D.
Intraocular pressure (IOP) in both eyes was measured 3 times by the same observer using a noncontact tonometer (Huvitz, HNT-7000). The mean value was calculated and taken as the final value for analysis.
Axial length (AL) was measured using optical biometry (Lenstar LS 900, Haag-Streit Diagnostics, Koniz, Switzerland) and the mean of five repeated measurements was recorded.
The iVue-100 SD-OCT (Optovue, Fremont, CA, USA) was used to measure the right eye of each subject. Images of ocular microstructures were obtained using a scanning laser diode emitting an 840 nm wavelength beam at a speed of 26,000 A-scans per second (16,17). The total scan time was 0.37 seconds per eye.
The protocol for the optic nerve head SD-OCT (iVue-100, Optovue) consisted of 12 radial scans (3.4 mm in length, 459 A-scans each) and 13 concentric ring scans (ranging from 1.3 to 4.9 mm, 429–969 A-scans each) centered on the optic disc (16,17). The areas between the A-scans were interpolated and various parameters were generated to describe the RNFL along a fixed 3.45-mm diameter ring centered on the optic disc. The RNFL values included: (I) average RNFL thickness; (II) temporal, superior, nasal, and inferior average RNFL thickness; (III) 16 sections (22.5° each) of the measurement circle around the optic nerve head (Figure 1); (IV) optic disc parameters including disc, cup, and rim area, cup/disc ratio, rim volume, nerve head volume, and cup volume.
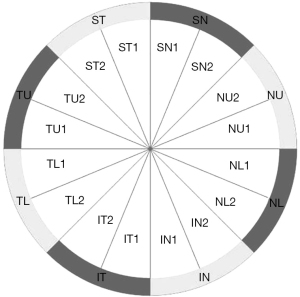
The GCC scan measured from the internal limiting membrane to the posterior boundary of the IPL and included: (I) average GCC—the average GCC thickness of the total measured area (Figure 2); (II) superior GCC—the average GCC thickness above the horizontal meridian; (III) inferior GCC—the average GCC thickness below the horizontal meridian; (IV) superior-inferior GCC (S-I GCC)—superior and inferior hemispheric difference of GCC thickness; (V) focal loss volume (FLV)—the average amount of focal loss over the whole GCC field; (VI) global loss volume (GLV)—the average amount of GCC loss over the whole GCC field.
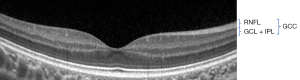
Image quality and integrity assessed with a signal strength index (SSI) higher than 45 (as recommended by the manufacturer) were labeled as good.
Statistical analysis
Statistical analysis was performed with Statistical Analysis System software (SAS 9.2; SAS Institute Inc., Cary, NC, USA). Only the data collected on the subjects’ right eyes were used for analysis. Continuous variables were compared using t-test and categorical variables were compared using Chi-squared test. Pearson’s correlation was used to evaluate the association of GCC with RNFL, AL, and SE. Statistical significance was considered as P<0.05.
Results
Demographics
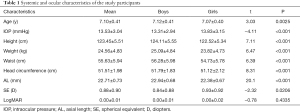
In total, 2,954 students underwent eye examinations, with 370 subjects excluded due to an SSI score below 45 and 79 subjects excluded because of a clinical diagnosis of amblyopia. A total of 2,505 subjects were enrolled in the study, including 1,452 boys (58.0%) and 1,053 girls (42.0%). The mean age was 7.10±0.41 (boys 7.12±0.41 vs. girls 7.07±0.40, P=0.0025). The data included age, IOP, height, weight, waist, head circumference, AL, and SE. The characteristics of the participants are summarized in Table 1.
Full table
Distribution of GCC
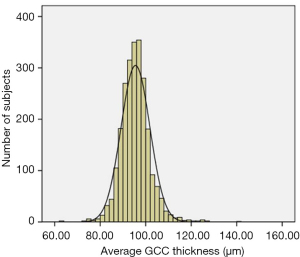
The mean GCC thickness was 95.31±7.67 µm (Figure 3). The superior GCC thickness (95.36±8.07 µm) was thicker than inferior GCC thickness (95.27±7.96 µm), although the difference was less than 1µm. The S-I GCC thickness was 0.10±4.59 µm, the GLV was 2.03%±2.41%, and the FLV was 1.24%±1.47%. The macular GCC parameters are presented in Table 2.
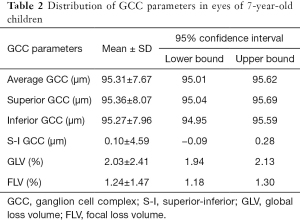
Full table
The average GCC thickness was 95.46±8.25 µm in boys and 95.11±6.80 µm in girls (P=0.2738). The parameters FLV, IT, TU2, ST1, SN1, IT1, IT2, and TL1 showed significant difference between boys and girls (Table 3).
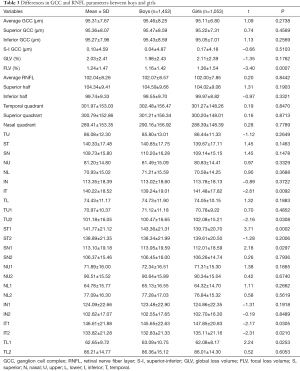
Full table
GCC thickness in the myopia, emmetropia, and hyperopia groups are shown in Table 4. Compared with myopic children, children with hyperopia had significantly thicker average GCC (95.69±7.45 vs. 93.33±9.83 µm), superior GCC (95.76±7.86 vs. 93.38±9.84 µm), and inferior GCC (95.61±7.67 vs. 93.35±10.35 µm). A difference in GLV was found between the hyperopia and emmetropia groups (1.95% vs. 2.31%, P=0.0075). There was no significant effect of refraction error on S-I GCC (P=0.5618), or FLV (P=0.5945).

Full table
Associations of GCC with ocular and systemic parameters
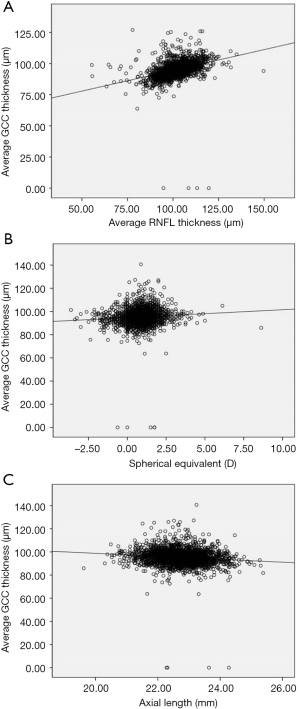
The relationships between GCC thickness and ocular and systemic parameters are shown in Table 5. Height, SE, RNFL parameters, fovea parameters, disc area, rim area, rim volume, and nerve head volume were found to have significant positive association with average GCC thickness, superior GCC thickness, and inferior GCC thickness. AL, area cup-to-disc (C-D) ratio, horizontal C-D (H C-D) ratio, and vertical C-D (V C-D) ratio were negatively correlated with average GCC thickness, superior GCC thickness, and inferior GCC thickness. Superior GCC thickness was associated with weight (P=0.0464). GCC thickness was not significantly associated with age, IOP, waist, head circumference, best corrected visual acuity (BCVA), cup area, or cup volume. Figure 4A,4B shows the positive relationships between RNFL thickness, SE, and average GCC thickness. Figure 4C shows the negative relationship between average GCC thickness and AL.
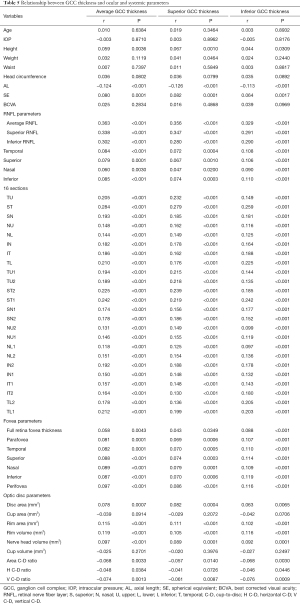
Full table
Discussion
Normal values of GCC thickness and the relationship with age, IOP, height, AL, SE, RNFL thickness, fovea, and optic disc were assessed in a large cohort of 7-year-old children in China. We found that the mean average GCC thickness was largely normally distributed, the superior GCC was thicker than the inferior GCC, and that GCC thickness was associated with AL, SE, RNFL thickness, height, fovea parameters, disc area, rim area, rim volume, nerve head volume, area C-D ratio, H C-D ratio, and V C-D ratio.
Consistent with the findings of previous studies (18,19), our study showed GCC thickness had a positive correlation with SE and negative correlation with AL. We also found that hyperopic children had thicker GCC than myopic children (P<0.0001), including superior GCC and inferior GCC (Table 4). GLV was lower in the hyperopia group compared to the emmetropia group (Table 4). However, in several studies, AL and SE (4) were found to have no correlation with GCC thickness. This might be due to the different age of participants (adults versus children), which may indicate that the results are impacted by age. Previous histopathologic studies have shown that increasing AL and eyeball expansion lead to the development of myopia (20). The elongation of the eyeball might result in mechanical stretching and traction, which may make the retina and sclera thinner in myopic eyes (21,22). The mechanical stretching is located mostly at the posterior pole and influences GCC and peripapillary RNFL (18). In addition, we found that S-I GCC and FLV had no correlation with SE. S-I GCC might be used as a parameter to track glaucoma in children; however, more data is needed to support the diagnostic performance of S-I GCC.
Our study demonstrated that GCC thickness had positive correlations with RNFL thickness, fovea parameters, disc area, and rim area and negative correlations with area C-D ratio, H C-D ratio, and V C-D ratio. RNFL parameters and optic disc parameters are useful for detecting glaucoma. However, optic disc tilting, peripapillary atrophy, and oval configuration in highly myopic eyes (23) may influence disc margin determination (24). The disc margin definition can affect RNFL and optic disc parameters, which are less reliable than GCC in the analysis of highly myopic eyes (25). GCC can be a marker for a decrease in RGCs, which can happen before visual field defects are apparent (26). Therefore, GCC may be superior to RNFL and optic disc parameters in the early detection of glaucoma progression (27).
We found that taller height was associated with thicker GCC. In a study of 42,044 participants, height was not associated significantly with GCC (P>0.30), which was measured using 3D OCT-1000 Mark II (Topcon Inc., Tokyo, Japan) (19). In a study of 258 children using the iScan OCT (Optovue), Grundy et al. found no evidence of a relationship between height or weight with either RNFL or GCC (28). These contradictory findings may be explained by differences in the ethnicity of subjects, sample size, and measurement equipment.
We observed no significant relationship between GCC thickness and IOP, which is consistent with previous studies (20,29). However, Khawaja et al. found a negative relationship between GCC thickness and IOP (P=5.8×10−5) (19). IOP above threshold will damage ganglion cells at the lamina cribrosa (30). Furthermore, it is possible that translamina cribrosa pressure difference (TLCPD, IOP minus cerebrospinal fluid pressure), and not IOP, is associated with the pathogenesis of glaucomatous optic neuropathy (31).
Age had no any significant association with GCC thickness, which is consistent with some previous studies (18,32). However, several studies have demonstrated a thinner retinal thickness with older age in adults (19,30), although this inconsistency may be due to our participants being young children. It would be useful to do a longitudinal study of GCC thickness and age in the future.
In our study, no significant differences were observed between girls and boys in average GCC thickness, superior GCC thickness, inferior GCC thickness, S-I GCC, or GLV. Similarly, Bloch et al. did not find sex differences in GCC thickness (30). However, women were found to have thicker macular inner retinas in the UK biobank (19) study and thinner superotemporal GC-IPL thickness in the Singapore Chinese Eye Study (SCES) (33). Although these studies found a significant association between inner retinal thickness and sex, their findings are based on a smaller sample size. Additionally, the correlation between GCC thickness and sex may vary among different ethnic groups. We found that BCVA, waist, head circumference, cup area, and cup volume were not correlated with GCC thickness, similar to the results of another report (28).
Our sample size of healthy Chinese children was large and the study was a school-based design, not population-based design. The schools were chosen to broadly represent the region. However, our study has several limitations that should be kept in mind. Our study only included children in the first grade and it would be beneficial to include child participants across a wide age span. By limitation of the ACES methodology, a noncontact tonometer was used to measure IOP rather than the Goldmann tonometer.
Conclusions
In conclusion, our study provides normative GCC thickness data, distribution patterns, and correlated ocular and systemic parameters in a large cohort of healthy 7-year-old children in China. The findings support an association between GCC thickness and AL, SE, height, RNFL, fovea parameters, disc area, rim area, rim volume, nerve head volume, area C-D ratio, H C-D ratio, and V C-D ratio in children. We did not find an association between GCC thickness and IOP, age, or sex. Future studies are planned to follow the cohort for further investigation of these associations.
Acknowledgments
The authors thank the Anyang government for helping to organize the survey.
Funding: This study was supported by the Major State Basic Research Development Program of China (‘973’ Program, 2011CB504601), the Major International (Regional) Joint Research Project of the National Natural Science Foundation of China (81120108007), the Capital Health Research and Development of Special Grant (2020-2-1081), Beijing Natural Science Foundation (JQ20029), the National Natural Science Foundation of China (82071000), Beijing Talents Fund (2016000021223ZK28), and the Beijing Nova Program (Z121107002512055).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-323
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tp-21-323
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-323). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Anyang Childhood Eye Study (ACES) was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University, Beijing, China (No. TRECKY2018-030). Each student provided verbal consent and informed written consent was obtained from at least one parent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang WW, Wang HZ, Liu JR, et al. Diagnostic ability of ganglion cell complex thickness to detect glaucoma in high myopia eyes by Fourier domain optical coherence tomography. Int J Ophthalmol 2018;11:791-6. [PubMed]
- Chen HS, Liu CH, Wu WC, et al. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci 2017;58:3637-45. [Crossref] [PubMed]
- Lee YP, Ju YS, Choi DG. Ganglion cell-inner plexiform layer thickness by swept-source optical coherence tomography in healthy Korean children: normative data and biometric correlations. Sci Rep 2018;8:10605. [Crossref] [PubMed]
- Kim NR, Lee ES, Seong GJ, et al. Comparing the ganglion cell complex and retinal nerve fibre layer measurements by Fourier domain OCT to detect glaucoma in high myopia. Br J Ophthalmol 2011;95:1115-21. [Crossref] [PubMed]
- Holló G. Optical coherence tomography angiography in glaucoma: analysis of the vessel density—visual field sensitivity relationship. Ann Transl Med 2020;8:1203. [Crossref] [PubMed]
- Naghizadeh F, Garas A, Vargha P, et al. Detection of early glaucomatous progression with different parameters of the RTVue optical coherence tomograph. J Glaucoma 2014;23:195-8. [Crossref] [PubMed]
- Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol 1982;100:135-46. [Crossref] [PubMed]
- Turk A, Ceylan OM, Arici C, et al. Evaluation of the nerve fiber layer and macula in the eyes of healthy children using spectral-domain optical coherence tomography. Am J Ophthalmol 2012;153:552-9.e1. [Crossref] [PubMed]
- Leung MM, Huang RY, Lam AK. Retinal nerve fiber layer thickness in normal Hong Kong chinese children measured with optical coherence tomography. J Glaucoma 2010;19:95-9. [Crossref] [PubMed]
- Altemir I, Pueyo V, Elía N, et al. Reproducibility of optical coherence tomography measurements in children. Am J Ophthalmol 2013;155:171-6.e1. [Crossref] [PubMed]
- Morales-Fernandez L, Jimenez-Santos M, Martinez-de-la-Casa JM, et al. Diagnostic capacity of SD-OCT segmented ganglion cell complex versus retinal nerve fiber layer analysis for congenital glaucoma. Eye (Lond) 2018;32:1338-44. [Crossref] [PubMed]
- Heidelberg Engineering Gmbh. Spectralis HRA+OCT with RNFL and ONH Normative Database. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf15/K152205.pdf
- Optovue, Inc.; RTVue with Normative Database. 510(k) Summary. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf10/K101505.pdf
- Chansangpetch S, Huang G, Coh P, et al. Differences in optic nerve head, retinal nerve fiber layer, and ganglion cell complex parameters between Caucasian and Chinese subjects. J Glaucoma 2018;27:350-6. [Crossref] [PubMed]
- Li SM, Liu LR, Li SY, et al. Design, methodology and baseline data of a school-based cohort study in Central China: the Anyang Childhood Eye Study. Ophthalmic Epidemiol 2013;20:348-59. [Crossref] [PubMed]
- Kang MT, Li SM, Li H, et al. Peripapillary retinal nerve fibre layer thickness and its association with refractive error in Chinese children: the Anyang Childhood Eye Study. Clin Exp Ophthalmol 2016;44:701-9. [Crossref] [PubMed]
- Zhu BD, Li SM, Li H, et al. Retinal nerve fiber layer thickness in a population of 12-year-old children in central China measured by iVue-100 spectral-domain optical coherence tomography: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci 2013;54:8104-11. [Crossref] [PubMed]
- Zhao Z, Jiang C. Effect of myopia on ganglion cell complex and peripapillary retinal nerve fibre layer measurements: a Fourier-domain optical coherence tomography study of young Chinese persons. Clin Exp Ophthalmol 2013;41:561-6. [Crossref] [PubMed]
- Khawaja AP, Chua S, Hysi PG, et al. Comparison of associations with different macular inner retinal thickness parameters in a large cohort: the UK Biobank. Ophthalmology 2020;127:62-71. [Crossref] [PubMed]
- Deng J, He X, Zhang B, et al. Increased vertical asymmetry of macular retinal layers in myopic Chinese children. Curr Eye Res 2019;44:225-35. [Crossref] [PubMed]
- Lam DS, Leung KS, Mohamed S, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci 2007;48:376-82. [Crossref] [PubMed]
- Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res 2006;82:185-200. [Crossref] [PubMed]
- Tong L, Chan YH, Gazzard G, et al. Heidelberg retinal tomography of optic disc and nerve fiber layer in Singapore children: variations with disc tilt and refractive error. Invest Ophthalmol Vis Sci 2007;48:4939-44. [Crossref] [PubMed]
- Leung CK, Cheng AC, Chong KK, et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci 2007;48:3178-83. [Crossref] [PubMed]
- Shoji T, Nagaoka Y, Sato H, et al. Impact of high myopia on the performance of SD-OCT parameters to detect glaucoma. Graefes Arch Clin Exp Ophthalmol 2012;250:1843-9. [Crossref] [PubMed]
- Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology 1992;99:19-28. [Crossref] [PubMed]
- Lavinsky F, Wu M, Schuman JS, et al. Can macula and optic nerve head parameters detect glaucoma progression in eyes with advanced circumpapillary retinal nerve fiber layer damage? Ophthalmology 2018;125:1907-12. [Crossref] [PubMed]
- Grundy SJ, Tshering L, Wanjala SW, et al. Retinal parameters as compared with head circumference, height, weight, and body mass index in children in Kenya and Bhutan. Am J Trop Med Hyg 2018;99:482-8. [Crossref] [PubMed]
- Cheng L, Wang M, Deng J, et al. Macular ganglion cell-inner plexiform layer, ganglion cell complex, and outer retinal layer thicknesses in a large cohort of Chinese children. Invest Ophthalmol Vis Sci 2019;60:4792-802. [Crossref] [PubMed]
- Bloch E, Yonova-Doing E, Jones-Odeh E, et al. Genetic and environmental factors associated with the ganglion cell complex in a healthy aging British cohort. JAMA Ophthalmol 2017;135:31-8. [Crossref] [PubMed]
- Wang N, Xie X, Yang D, et al. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology 2012;119:2065-73.e1. [Crossref] [PubMed]
- Kita Y, Naghizadeh F, Kita R, et al. Comparison of macular ganglion cell complex thickness to total retinal thickness ratio between Hungarian and Japanese eyes. Jpn J Ophthalmol 2013;57:540-5. [Crossref] [PubMed]
- Koh VT, Tham YC, Cheung CY, et al. Determinants of ganglion cell-inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:5853-9. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


