Editor’s Note:
In the era of personalized medicine, a critical appraisal new developments and controversies are essential in order to derived tailored approaches. In addition to its educative aspect, we expect these discussions to help younger researchers to refine their own research strategies.
Cons: the confusing mucinous adenocarcinoma classification
Introduction
In 2015 a new WHO classification of lung tumors was issued. This classification clarified several important issues and corrected many entities (1). For adenocarcinomas the previous published classification (2) was adapted and largely taken over. Adenocarcinomas are now grouped into non-mucinous adenocarcinomas and adenocarcinoma variants. Within the non-mucinous adenocarcinomas the evaluation of primary, secondary, and tertiary patterns have to be given in percentages; patterns recognized are lepidic, acinar, papillary, micropapillary, and solid. Recently cribriform pattern was added as another pattern conferring worse prognosis (3,4). Patterns are also important for prognosis, as lepidic pattern confers a good prognosis, whereas solid and micropapillary regardless of their percentage confers a worse prognosis.
For the preinvasive lesions non-mucinous (NMAC) as well as mucinous adenocarcinoma (MAC) in situ are well accepted (1), whereas in the invasive adenocarcinomas mucinous variants have been handled like an orphan. Several aspects are confusing:
Mucin production in NMAC is accepted: “the neoplastic cells and/or glandular spaces may contain mucin”, but to what extent has never been clarified. Non-mucinous solid adenocarcinomas are classified by “mucin present in ≥5 tumor cells in 2 HPF”. What is the upper limit? How much mucin production is allowed in a NMAC before changing the name to mucinous adenocarcinoma?
Questions and discussion
Let us switch to the definition of mucinous adenocarcinomas for a hope of clarification.
Under variants we are faced with two entities: invasive mucinous adenocarcinoma (IMAC) and colloid adenocarcinoma, both are mucin producing.
(I) Starting with the definition we learn, that IMAC includes cases of mucinous bronchioloalveolar adenocarcinoma (MBAC) and cases of mucinous adenocarcinoma with goblet cell and columnar morphology.
MBAC has been defined as non-invasive adenocarcinoma since the WHO classifications 1999 and 2004 (5,6). So MBAC by this definition are clearly mucinous AIS. Therefore this does not need to be further discussed. Up to now there are still reports using the term bronchioloalveolar adenocarcinoma despite the changes of the classification, and there were reports allowing invasion to be part of the MBAC diagnosis. But the only reaction is to not include these reports into any study, as the survival data cannot be compared to any other study.
(II) When looking for a definition of mucin production we find this statement: “abundant intracytoplasmic mucin”. So what is abundant? In addition there is a statement, that any growth pattern except solid may be seen, lepidic being the most common. This raises the question why IMAC has not been subclassified similar to NMAC into predominant lepidic, acinar, papillary, micropapillary, and cribriform?
With respect to patterns there are two statements are conflicting and potentially misleading: “The term lepidic predominant adenocarcinoma should not be used in the context of invasive mucinous adenocarcinoma with predominant lepidic growth” (p34). And: “The tumor may show the same heterogeneous mixture of lepidic, acinar, papillary and micropapillary growth as the non-mucinous tumors”… “Although invasive mucinous adenocarcinoma often shows lepidic predominant growth. Extensive sampling usually reveals invasive foci…” (p39).
It seems the authors of this classification have overlooked these discrepancies in attempting to propose a uniform terminology. These couple of examples illustrate lack of clarity for the proposed criteria and terminology: lepidic adenocarcinoma is an invasive adenocarcinoma, therefore a lepidic mucinous adenocarcinoma is invasive by this name. It is not necessary to state this. Extensive sampling is necessary in every lepidic adenocarcinoma, as it is important to prove invasion otherwise it would be a mucinous or non-mucinous AIS or MIA, which change the prognosis substantially. It is usually argued that the clinical impact of subclassifying IMAC by pattern has not been proven. Given the relative rarity of IMAC compared to NMAC might leave this question unresolved for quite a while. Why not contrary use this subtyping and investigate the impact retrospectively in case series, as it has been done for NMAC? Studies showing the value and reproducibility of the NMAC subclassification appeared after the publication of the classification. So why not in IMAC?
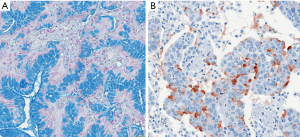
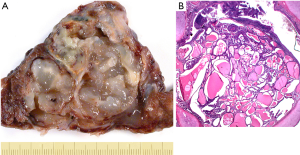
(III) Quantification of mucin. This is a complicated issue. As with many secretory products the tumor cells synthesize mucin, store mucin in specialized cytoplasmic vesicles, secrete mucin, and enter a refractory period. Whereas in normal mucin producing cells of the respiratory tract, this is synchronized and tightly regulated by proteins such as EGFR, interleukin receptor 4α, and MUC genes (7-10), in tumors this process is not anymore synchronized (11-13). This results in a morphologic spectrum of cells with stored mucin, cells that are releasing mucin, and cells with minimal residual mucin (resting cells). In addition some cells used apocrine secretion (vesicles with mucin are released), some holocrine secretions (mucin with apical components of the cells are released), which result in change of the cells shape. From our own experience in IMAC usually >70% of tumor cells will show mucin, which will be best highlighted by a mucin stain (Figure 1) or by immunohistochemistry for one of the MUC proteins. However, it should be mentioned, that staining for MUC proteins is less specific (see below). A similar picture is seen in other organs with secretory cells such as the thyroid: cell undergoes a constant change from synthesis–storage–release–resting and the cells change their shape accordingly. In thyroid carcinomas, especially papillary carcinomas again the synchronization is lost. The decision on how much mucin is necessary to call the tumor IMAC is also complicated by the presence of mixed NMAC-IMAC cases. This requires setting a lower and upper limit of mucin in a given carcinoma. Based on my own experience the combined NMAC-IMAC cases are usually composed of areas of NMAC and adjacent areas of IMAC. So there are always definite areas, which enable a diagnosis. The question therefore needs to be rephrased: How much percentage of each component is necessary to create an IMAC or a mixed NMAC-IMAC? And how much mucin is accepted in a NMAC?
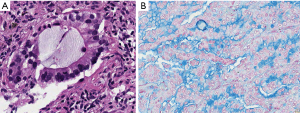
In carcinogenesis the starting point are several cells or cell clones coming from precursor cells and thus having capacities for differentiation toward cells of the normal mucosa. This means that in adenocarcinomas the differentiation program for ciliated, goblet, secretory columnar, Clara, and pneumocytes can be active. However, it is also well known that in any given carcinoma usually a few clones take the lead. So scattered single cells with mucinous differentiation are acceptable in NMAC, whereas mucin-producing cells dominate IMAC (Figure 2). Given the percentage of mucin producing cells as outlined above IMAC might be separated from NMAC more precisely. For mixed NMAC-IMAC the definition might be areas of at least 10% of either NMAC or IMAC within an adenocarcinoma might be acceptable. And each component should form a compact area.
(IV) Is there any help by looking up types of mucin for discrimination between IMAC and NMAC? Is there a difference in the genetic profile of these adenocarcinomas?
There are several MUC proteins expressed by cells of the bronchial tree and the alveolar cells. MUC2, MUC5AC, MUC5B, MUC6 map to 11p15.5 and encode secretory gel forming mucins while MUC1, MUC3, MUC4, MUC7 are scattered on different chromosomes and encode membrane-bound or secreted mucins. Mucus-producing cells mainly express MUC5AC and MUC5B whereas MUC4 is expressed in a wide array of epithelial cells. MUC5AC besides overexpressed in metaplasia, dysplasia and normal epithelium adjacent to squamous cell carcinoma is also related to mucus formation in well differentiated adenocarcinomas especially with goblet cell morphology (14). MUC-1 glycoprotein is frequently expressed by mucin-producing adenocarcinomas except the goblet cell-type. Therefore expression of MUC1, MUC2 and MUC5ABC has some value assisting in the diagnosis of mucinous adenocarcinomas, especially MUC5 for the adenocarcinomas with basolateral mucin secretion such as in colloid and cystadenocarcinomas. However MUC1/2 expression is more specifically associated with colloid and signet ring cell types of adenocarcinomas. MUC 1 is found in almost every type of mucinous adenocarcinoma. So overall the staining for MUC proteins is not overwhelmingly reliable (15,16). The rarity of MUC 2 in IMAC might be best explained by an article showing that CDX2 is a transcriptional regulator of MUC2 (17). In one study the expression of depolarized MUC1, MUC5AC, and MUC6 were correlated significantly with p53 gene mutations and tumor size (18).
Does TTF1 help in sorting IMAC?
In our study almost one third of IMACs were negative for TTF1. Several of these cases were also negative for CDX2 (13). In another study it was shown that HNF4α might replace TTF1 in these tumors (19). So staining for HNFα should be investigated in IMAC versus NMAC to show its value as a differentiation marker.
Is there a role for specific genetic aberrations?
KRAS mutation was found in 56% of mucinous adenocarcinomas. Mutational status was neither correlated with architectural pattern nor survival. Codon 12 mutations were most frequent, and one case presented with KRAS mutations in codon 12 and 61. Goblet cell variants of mucinous adenocarcinomas presented predominantly with codon 12 mutations, while all colloid variants had KRAS mutation (15). This was confirmed by another study which possibly due to the small number of cases found only codon 12 mutations (16). So KRAS mutation is of no help for the differential diagnosis too. ALK gene fusions are as rare as these are in NMAC. Recently Neuregulin fused to different other genes was identified as a new driver mutation in IMAC. This fusion oncogene can concur with KRAS mutation but also with ALK rearrangements. However, this fusion was seen in approximately 20% of IMAC and thus might not be useful for a differential diagnosis, and as this was not compared to NMAC it might be useful as a therapeutic target but not for differential diagnosis (20).
(V) In the classification the importance of columnar and goblet cells is mentioned. But there is no explanation why this is of importance? Usually in IMAC all cells are columnar, some high some more cuboidal; most often this reflects the state of mucin production: A cell full of mucin is usually high columnar, whereas a cell in resting is cuboidal (Figure 2). Goblet cell morphology on the other hand is associated with the type of secretion of mucin: Goblet cells in carcinomas as well as normal goblet cells secrete mucins in an apical fashion, sometimes also in a holocrine fashion, i.e., portions of the secretary vacuole is released together with the mucin (Figure 3). Other columnar cells most likely derived from secretary cells of the mucosa release mucin also apical, but without cellular content. A rare mucinous adenocarcinoma is derived from bronchial glands, is usually acinar and composed of a mixture of goblet and secretory cells. These adenocarcinomas are located centrally. The only non-columnar cells in mucinous AC are the signet ring cell and cells from colloid carcinomas, and these are characterized by a non-directed random mucin secretion (Figure 4). These cells have lost the basoapical orientation and therefore can release mucins towards the basal membrane or laterally. This has also to do with a downregulation of adhesion molecules and the ability to easily detach from cell complexes.
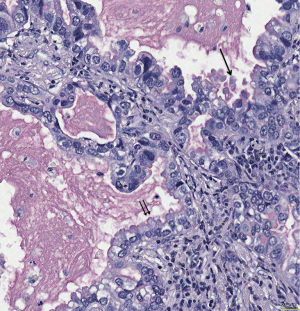
(VI) Until recently in the classification of solid adenocarcinomas the proof of mucin production was required in at least a minority of cells. Despite that solid adenocarcinoma was placed within the non-mucinous types. But mucin production is a sign of differentiation, as it requires a sequence of signaling events to enable the cell for this service. Usually in undifferentiated carcinomas cytoplasmic features and synthesis of substances which are characteristic of normal cells is gradually lost (21). So the synthesis of mucin in a minority of cells is still a sign of differentiation, whereas the suppression of mucin synthesis in the majority is a sign of dedifferentiation. In essence these solid adenocarcinomas belong to the mucinous variants, either as a separate entity or under the header of invasive mucinous adenocarcinomas. In the new WHO classification another solid adenocarcinoma appeared: solid AC without mucin but TTF1 positive. This might be the counterpart to the mucinous solid adenocarcinoma, as it is not programmed to synthesize mucins. If these two forms of solid adenocarcinomas have other molecular differences has to be investigated.
(VII) Signet ring cell, colloid adenocarcinoma, cystadenocarcinoma are no longer separate entities—is there a scientific evidence for that?
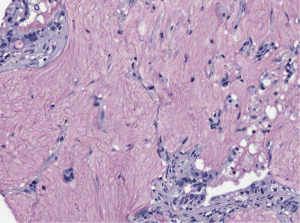
Colloid adenocarcinoma is characterized by a diffuse infiltration of the lung, abundant mucin secretion and a dramatic spillover of mucin in adjacent segments/subsegments even in small carcinomas. This requires careful surgical resection with many frozen section for the resection margins to rule out spreading tumor cells. Cystadenocarcinoma is morphologically similar to colloid adenocarcinoma, but has usually a fibrous pseudocapsule and the resection is easier. For cystadenocarcinoma a precursor lesion is known, the mucinous cystadenoma, and the mucinous borderline lesion (a mucinous cystadenocarcinoma in situ; Figure 5) (22-24). So far there is not such a precursor for the colloid adenocarcinoma. Colloid adenocarcinomas are usually KRAS mutated, the mutation status in cystadenocarcinomas as well as in cystadenomas has not been evaluated in larger series.
In the 2015 WHO classification signet ring cells should be mentioned if they occur within IMAC. However, how to call an adenocarcinoma entirely composed of signet ring cells? What reason is there to skip the term signet ring cell carcinoma (SRCC)?
In one study it was shown, that MUC1, TTF1, and cytokeratin 7 is highly expressed in SRCC in contrast to other MUCs in IMAC (25). SRCC more often have ALK rearrangement and are associated with EGFR or KRAS (26). Mucin-histochemistry showed a close similarity between lung SRCC and goblet cell-type or bronchial gland cell-type adenocarcinoma of the lung. Lactoferrin, a marker of bronchial gland cell differentiation is significantly associated with SRCCs, suggesting that this type of adenocarcinoma might be closely related to bronchial gland type adenocarcinoma (27). In cases of adenocarcinomas with a minor population of signet ring cells, this should be mentioned, similar as minor structural components are mentioned.
In summary invasive mucinous adenocarcinomas should be subtyped by pattern similar to NMAC; solid adenocarcinoma with mucin producing cells should be placed into IMAC, whereas the non-mucinous solid adenocarcinoma with TTF1 positivity should remain in NMAC. Cell types should be mentioned within the IMAC classification as being either a predominant or a minor component. Cystadenocarcinoma should be separated from colloid adenocarcinoma, and signet ring cells carcinoma should remain as a separate entity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition ed. WHO Classification of Tumours. Geneva: IARC, WHO Press, 2015.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski C, et al. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol 2015;10:638-44. [Crossref] [PubMed]
- Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [Crossref] [PubMed]
- Travis W, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleura Tumours. 3rd ed. WHO, International Histological Classification of Tumours. Berlin, Heidelberg, New York: Spinger; 1999.
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC WHO Classification of Tumours. Lyon: IARC Press, 2004:10.
- Temann UA, Prasad B, Gallup MW, et al. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol 1997;16:471-8. [Crossref] [PubMed]
- Takeyama K, Dabbagh K, Lee HM, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A 1999;96:3081-6. [Crossref] [PubMed]
- Zuhdi Alimam M, Piazza FM, Selby DM, et al. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol 2000;22:253-60. [Crossref] [PubMed]
- Gray T, Koo JS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 2001;160:35-46. [Crossref] [PubMed]
- Seregni E, Botti C, Lombardo C, et al. Pattern of mucin gene expression in normal and neoplastic lung tissues. Anticancer Res 1996;16:2209-13. [PubMed]
- Nguyen PL, Niehans GA, Cherwitz DL, et al. Membrane-bound (MUC1) and secretory (MUC2, MUC3, and MUC4) mucin gene expression in human lung cancer. Tumour Biol 1996;17:176-92. [Crossref] [PubMed]
- Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch 2015;467:675-86. [Crossref] [PubMed]
- Copin MC, Buisine MP, Devisme L, et al. Normal respiratory mucosa, precursor lesions and lung carcinomas: differential expression of human mucin genes. Front Biosci 2001;6:D1264-75. [Crossref] [PubMed]
- Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch 2015;467:675-86. [Crossref] [PubMed]
- Maeshima A, Miyagi A, Hirai T, et al. Mucin-producing adenocarcinoma of the lung, with special reference to goblet cell type adenocarcinoma: immunohistochemical observation and Ki-ras gene mutation. Pathol Int 1997;47:454-60. [Crossref] [PubMed]
- Mesquita P, Almeida R, Van Seuningen I, et al. Coordinated expression of MUC2 and CDX-2 in mucinous carcinomas of the lung can be explained by the role of CDX-2 as transcriptional regulator of MUC2. Am J Surg Pathol 2004;28:1254-5. [Crossref] [PubMed]
- Awaya H, Takeshima Y, Yamasaki M, et al. Expression of MUC1, MUC2, MUC5AC, and MUC6 in atypical adenomatous hyperplasia, bronchioloalveolar carcinoma, adenocarcinoma with mixed subtypes, and mucinous bronchioloalveolar carcinoma of the lung. Am J Clin Pathol 2004;121:644-53. [Crossref] [PubMed]
- Kunii R, Jiang S, Hasegawa G, et al. The predominant expression of hepatocyte nuclear factor 4alpha (HNF4alpha) in thyroid transcription factor-1 (TTF-1)-negative pulmonary adenocarcinoma. Histopathology 2011;58:467-76. [Crossref] [PubMed]
- Shin DH, Lee D, Hong DW, et al. Oncogenic function and clinical implications of SLC3A2-NRG1 fusion in invasive mucinous adenocarcinoma of the lung. Oncotarget 2016;7:69450-65. [PubMed]
- Creighton C, Hanash S, Beer D. Gene expression patterns define pathways correlated with loss of differentiation in lung adenocarcinomas. FEBS Lett 2003;540:167-70. [Crossref] [PubMed]
- Kragel PJ, Devaney KO, Meth BM, et al. Mucinous cystadenoma of the lung. A report of two cases with immunohistochemical and ultrastructural analysis. Arch Pathol Lab Med 1990;114:1053-6. [PubMed]
- Gao ZH, Urbanski SJ. The spectrum of pulmonary mucinous cystic neoplasia: a clinicopathologic and immunohistochemical study of ten cases and review of literature. Am J Clin Pathol 2005;124:62-70. [Crossref] [PubMed]
- Monaghan H, Salter DM, Ferguson T. Pulmonary mucinous cystic tumour of borderline malignancy: a rare variant of adenocarcinoma. J Clin Pathol 2002;55:156. [Crossref] [PubMed]
- Tsuta K, Ishii G, Nitadori J, et al. Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol 2006;209:78-87. [Crossref] [PubMed]
- Kadota K, Yeh YC, D'Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol 2014;38:1118-27. [PubMed]
- Hayashi H, Kitamura H, Nakatani Y, et al. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol 1999;30:378-83. [Crossref] [PubMed]


