Management of gastric cancer in Indian population
Introduction
Adenocarcinoma of the stomach ranks 2nd globally in cancer related death. Globally, it accounts for 989,600 new cases and 738,000 deaths annually. The case-mortality rate is high in gastric cancers than other common malignancies like breast, colonic and prostatic cancers (1). Even in this era, the disease is commonly identified only after the muscularis propria invasion, because of the vague symptoms at earlier stages as the classical symptoms of anemia & cachexia is seen only in late stages. Role of surgery and chemotherapy is only for palliation at late stages and role of targeted therapy is still at an early era. Gastric malignancies have poor prognosis and a 5-year survival rate of 20 per cent. Gastric cancer clinical presentations vary among other countries and race. A review about its presentation and management in India is discussed here.
Epidemiology
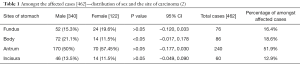
The gastric cancer incidence in India is relatively low compared to global incidence. The age-adjusted rate (AAR) for gastric cancer in India is 3.0–13.2 compared to global AAR 4.1–95.5 (1). Worldwide incidence of gastric cancer is on a decline due to factors like hygienic food, sanitation, and food preservation techniques. Such a decline is not seen in certain parts of India (1). In India the gender based cancer incidence is more common in south Indian males than the north Indian males, which occurs a decade later. A West Bengal based study reveals antrum as the most common site of involvement (51.9%) followed by body (18.6%) and fundus (16.5%) and the least being incisura (12.9%). And it has also revealed that body mucosa was significantly involved in males than in females (see Table 1) (3). Variations in the dietary pattern, tobacco and alcohol usage increases the risk considerably. In Trivandrum, a case-control study revealed higher temperature foods, rice and chili as independent risk factors for gastric cancer. A Hyderabad based study compared 94 gastric cancer patients and 100 normal age- and sex-matched controls, smoking (P<0.01) and alcohol (P<0.05) were significantly associated with gastric cancer (4).
Full table
In India, Mizoram has been reported to have the highest gastric cancer incidence. The AAR in males and females has been reported at 50.6 and 23.3 (5). Hospital-based data from Mizoram showed that most common cancer accounting for 30% of all cancer cases is gastric cancer. The male-to-female ratio was 2.3:1; the average age for female was 57 years and male was 58 years.
Etiology
The highest gastric cancer incidence in Mizoram is due to dietary and possibly some unknown genetic differences. In a case–control study from Mizoram among the cases, gastric cancer risk is high in smokers [odds ratio (OR), 2.3; 95% confidence interval (CI), 1.4–8.4]. Meiziol (a local cigarette) smokers (OR, 2.2; 95% CI, 1.3–9.3) showed higher risks. Tuibur (tobacco smoke-infused water), very commonly used in Mizoram, was associated with the risk of stomach cancer among current users in both univariate and multivariate models (OR, 2.1; 95% CI, 1.3–3.1).
In a Chennai based study, alcohol and pickled food intake were identified as independent risk factors for gastric cancer. On the other hand, use of pulses was found to be offering a protective effect. Hospital-based data are prone to selection and referral bias and hence the above results need to be viewed with caution.
In another hospital-based study from Kashmir, there was no association found between gastric cancer and Helicobacter pylori infection in 1,314 patients. Similar to Mizoram, the incidence was higher in males and the cancer occurred most commonly in middle aged patients. The most common site for tumor was body of stomach (40.7%) followed by the pylorus (35.5%). In conclusion, the epidemiology of gastric cancer suggests that it is not a single disease or caused by a single factor, but a combination of genetic, sociocultural, and environmental factors in a given region dictates its presentation. Gastric cancer can broadly be classified as intestinal or diffuse as proposed by Lauren et al. based on histological findings (6). Based on the anatomic site it is classified as proximal (cardia, fundus, and gastroesophageal junction) and distal (pylorus).
Interestingly, the incidence of proximal cancers is increasing in the developed world in concordance with the increase in esophageal cancers suggesting that these might have similar risk factors and pathologies (6). H. pylori, a Gram-negative bacteria, is associated with gastric mucosal infection. In underdeveloped countries with poor hygienic conditions, 50–90% of the population is infected asymptomatically in childhood. H. pylori has been attributed to cause distal gastric cancers and it is believed that the overall decline in gastric cancers and more so distal cancers worldwide is due to reduction and eradication of H. pylori infection with improved sanitation. However, it should be assumed that countries with a very high prevalence should have the highest incidence but this is not true as Asia and Africa although with a higher incidence for H. pylori infestation have a lower gastric cancer incidence. This Asian or African paradox suggests that H. pylori by itself cannot cause gastric cancer and various other factors are needed for causation. It is also believed that the poor study design and inaccuracies in techniques quantifying H. pylori may account for such paradoxical results. Various etiological factors including smoking, alcohol, nitrates, and H. pylori have been proposed as gastric cancer causes in India.
Pathology
In a study in a tertiary care center in Mysore, over 83 endoscopic gastric biopsies and gastrectomy specimens received from department of surgery during the period January 2009 to May 2011 were included in the study. The results showed Gastric carcinoma was most common in males and in the 7th decade. Most common histological type of tumor was intestinal type 78.3%, seen in the 7th decade followed by diffuse type 21.7% in the 6th decade. Precursor lesions were positive in out of 70 cases of gastric carcinoma. And chronic atrophic gastritis was found in 42% of cases, dysplasia in 33% and intestinal metaplasia in 36%. H. pylori were positive in 54.3% cases; among them 56.4% cases were intestinal type of carcinoma and 46.6% were diffuse type of carcinoma. A West Bengal based study shows ulcerative lesion (57.8%) was significantly common as compared to ulceroproliferative (24.9%) and polypoidal lesion (17.3%) (see Table 2)
Pathogenesis
Helicobacter pylori
H. pylori is a potential risk factor for gastric carcinogenesis leading to numerous researches into the mechanisms involved. The virulence of organism, a permissive environment, and a genetically susceptibility are the key factors for H. pylori-related gastric carcinogenesis. H. pylori triggers a sequence of events that facilitate the normal gastric epithelium conversion to atrophic gastritis, intestinal metaplasia, and dysplasia to carcinoma. The H. pylori secretes urease, protease, phospholipase, ammonia, and acetaldehyde which causes mucosal damage and further disrupts gastric protective functions via urease-mediated myosin II activation.
Production of oxidative stress keys in for virulence in H. pylori-infected by producing reactive oxygen and nitrogen species which suppresses the defense mechanisms, resulting in DNA damage. H. pylori supports the formation of mutagenic substances through inflammatory mediators or by impairing the mismatch repair pathway. Kim et al. revealed that H. pylori induces the cascade of carcinogenesis by promoting internal DNA damage while decreasing repair capabilities and mitochondrial and nuclear DNA mutations. Aberrant DNA methylation is also reported as risk factor (7).
Gene profiling for H. pylori infected patients done by PCR assays have demonstrated variable expression of up to 38 genes, which points out H. pylori infection leads to host defense evasion, enhancement of inflammatory and immune responses, activation of NF-κB and Wnt/β-catenin signaling pathways, perturbation of metal ion homeostasis, and induction of carcinogenesis.
Dietary factors
Around 2,000 epidemiological and experimental studies have revealed the role of dietary factors in gastric carcinogenesis. Starch rich and low protein diet consuming population are at higher risk as they favor acid-activated nitrosation in the stomach which leads to gastric mucosa damage. In Korea using an ecological approach, Park et al. (8) demonstrated lower risk for mortality in refrigerator users and of fruit intake compared to a higher risk in salt users.
D’Elia et al. reported increased risk due to increasing dietary salt intake. Salty food favors H. pylori colonization and which further induces cascade of events leading to carcinogenesis.
Dietary nitrates are found in vegetables and as preservatives and fertilizers. Gastric acid breaks down dietary nitrate into carcinogenic N-nitroso compounds (NNC). Preformed NNC and nitrosamines may also be present in smaller quantities in cured meats, dried milk, instant soups, and coffee dried on direct flame.
Cooking practices like meats roasting, broiling, grilling, baking and deep frying in open furnaces, sun drying, salting, curing, and pickling, lead to increase NNC. In smoked food, polycyclic aromatic hydrocarbons such as benzo(a)pyrene have been detected which increases the gastric cancer risk considerably.
Lifestyle
A population-based prospective cohort study revealed increased gastric cancer risk in consumption of alcohol and tobacco. Smokers tend to have a higher incidence of H. pylori infection and gastroduodenal inflammation than non-smokers. Smoking history is a significant independent risk factor for death from gastric cancer in patients who had undergone curative surgical resection. Smoking decreases prostaglandins, which protects integrity of the mucosa leading to the development of precursor lesions such as gastritis, ulceration, and intestinal metaplasia.
Clinical features
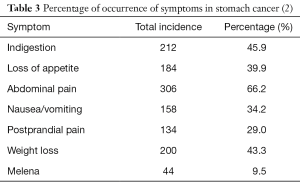
Kabir et al. stated abdominal pain (100%), vomiting (78%), dysphagia (24%), and weight loss (62%) as predominant symptoms while; Qurieshi et al. stated weight loss (35%), dyspepsia (76%), anorexia (35%), and vomiting (35.8%) predominantly. Our study showed that abdominal pain (66.2%) was the commonest symptom followed by weight loss (43.3%), indigestion (45.9%), anorexia (39.9%), nausea/vomiting (34.2%), postprandial pain (29%), and melena (9.5%). Our findings were similar to the findings of the study done by Qurieshi et al. Our cohort study showed that the obstructive symptoms like postprandial abdominal pain, nausea/vomiting, and weight loss were common in fundal and obstructive variety of antral carcinoma, which was not shown in any study after thorough Medline search (2). Comparison of presenting symptoms from the West Bengal based study is shown in Table 3. Abdominal pain (66.23%), indigestion (45.88%) and weight loss (43.29%), and the least was melena (9.52%).
Several studies report progressive increase in proximal cancer and concomitant decline in distal cancer in the west. But Asian reports suggested otherwise. Non cardia cancer incidence is more in Japanese & Korean population whereas an Iranians predominantly had cardia cancer. In our study antrum was mostly involved (51.9%), body (18.6%) and fundus (16.4%). Gastric body was significantly involved in males. Afridi et al. showed that cancer incidence in pylorus and antrum is 40%, cardiac end is 33%, linitis plastica is 13.3%, and body and pylorus is 6.7% (2). A South Indian study from Kerala suggested predominance of cancer in antrum & progressing trend towards proximal stomach. In Kashmiri population Qurieshi et al. showed that cancer incidence in proximal, mid, and distal stomach were 42%, 6.2%, and 45.7% (2).
Staging and diagnosis
Till date diagnostic modality of gastric cancer is flexible upper GI endoscopy and biopsy. Usually an 8 core biopsy technique is followed with 4 biopsies superficially and 4 biopsies taken deeper from the same site. Linitis plastica most of the times challenges this technique where still deeper biopsies may be needed. The poor distensibility of stomach during upper GI endoscopy will give a clue about possible Linitis Plastica. Gastric lymphoma is considered as a differential diagnosis if the histology is poorly differentiated. In that case Immuno Histo Chemistry comes to our rescue.
The staging is usually done with USG abdomen and Contrast Enhanced Computed Tomography. The details looked are for distant metastasis, extent of invasion, D1 spread, plane with pancreas, gastric outlet obstruction, nodal stations involved, residual stomach volume post resection and need for any composite resections. PET CT is not routinely recommended except in Special case with doubtful diagnosis or in suspicious metastasis. False negative PET CT is common in mucin secreting signet ring cell adeno carcinoma due to GLUT-1 transporter deficiency. Gastric signet ring cell carcinoma (GSRC) is known to have low fluorodeoxyglucose (FDG) uptake (9).
Staging laparoscopy is performed prior to definitive surgery. In a study, staging laparoscopy in 40 patients revealed more accurate staging of the disease and certainty of resectability. Study revealed unresectability in 11 cases (27.5%) and M1 in 5 cases (12.5%). Thus staging laparoscopy benefitted 16 patients (40%) from laparotomy and confirmed the feasibility of resection in 24 patients, and this finding was borne out in 20 of 23 patients surgically explored (87%). Hence staging laparoscopy holds a 91.6% diagnostic accuracy rate with no morbidity or mortality. We conclude staging laparoscopy as an effective tool prior to definitive surgical resection (10).
Treatment
Surgery
Gastrectomies with nodal dissection is the standard of treatment for gastric cancer. Based on site Subtotal gastrectomy in antral cancers and total or proximal gastrectomy in fundus or body is preferred (11). A tumor-free resection margin of at least 4 cm is needed for the adequacy of the surgery. Patients are considered surgically unresectable if there is evidence of metastasis or locoregional spread involving the peritoneum or encasement of major vessels. There is no role of debulking surgery in gastric cancer (11). There is a considerable controversy regarding the role and extent of lymphadenectomy in gastric cancer. Extensive lymphadenectomy also called as D2 dissection is widely practiced in far eastern countries like Japan and Korea. The survival advantage and decreased mortality seen with D2 lymphadenectomy by Japanese surgeons has not been translated in Western countries. Data from Europe and USA have shown that more conservative D1 lymphadenectomy is equal to D2 lymphadenectomy in terms of overall survival with lesser morbidities (12). A middle path approach of less aggressive D2 lymphadenectomy also called as modified D2 lymphadenectomy excludes splenectomy and pancreatectomy and has been found to be equivalent to D2 lymphadenectomy. Surgical skills and the volume of surgery done in a center also influence the outcomes of lymphadenectomy; results with D2 lymphadenectomy are better in Japan because of more experienced surgeons and large volume of surgeries for gastric cancer. For an adequate pathological staging a specimen with at least 15 lymph nodes is a must. A study from Tata Memorial Hospital, Mumbai, has shown D2 lymphadenectomy to be safe with outcomes comparable to Japanese studies. The study included 159 patients with resectable tumors operated with D2 Gastrectomy (13). Median number for lymph nodes dissected was 15 and the rate of major morbidity was 4.4% with the mortality due to surgery being 1.25%. This study shows that Indian surgeons in high-volume centers can achieve results comparable to best centers in the West.
In inoperable disease with gastric outlet obstruction a palliative bypass, anterior Gastro jejunostomy is done to palliate the symptoms. If the bypass is not feasible due to extensive gastric involvement, a feeding jejunostomy is done to maintain the nutrition of the ailing patient. In case of Gastric Outlet Obstruction with poor general condition, who may not be fit for any surgical procedure, an endoscopic stenting can be attempted.
Chemotherapy
Chemotherapy is gaining momentum in the management of gastric cancers. A significant R0 resected gastric cancer patients relapse with locally and distant metastases, suggesting that gastric cancers tend to metastasize early in the course of the disease. Chemotherapy can be used prior to surgery to shrink the tumor and make it operable; this is called neoadjuvant chemotherapy (NACT). Chemotherapy can also be given in the adjuvant setting after complete surgical resection. The only randomized control trial (RCT) on perioperative chemotherapy was the MAGIC trial conducted by the Medical Research Council, UK (14), randomized 503 patients with gastrectomies alone and gastrectomies combined with perioperative chemotherapy with 5-flurouracil epirubicin and cisplatinum (ECF). Patients who received perioperative chemotherapy had better overall survival and progression-free survival in comparison to patients who underwent surgery alone. Twenty-five percent of patients in the study had cancer of the gastroesophageal junction or lower esophagus. There are very little data from RCT in adjuvant therapy. A Japanese study has showed improved survival with S1, a prodrug combining fluoropyrimidine (tegafur) with oxonic acid, in patients with stage 2 and 3, completely resected gastric cancer. The drug is not easily available outside Japan. Significant proportion of Indian patients present with unresectable or advanced lesions. These patients have an incurable disease and the role of chemotherapy in them is purely palliative. Various chemotherapeutic agents have shown an activity in gastric cancer; typically, these agents have a response rate of 10–20% when used individually. The commonly used chemotherapy drugs are cisplatinum, 5-flurouracil, capecitabine, paclitaxel, epirubicin, docetaxel, paclitaxel, oxaliplatin, and irinotecan. Chemotherapy combinations are preferred to single agents for a faster response, although toxicity increases with the use of combination chemotherapy. Chemotherapy in metastatic gastric cancer holds a better survival rate than TLC. However, the relative survival rate with various chemotherapy combinations or single agents has ranged from 9 to 11 months. Significant proportions of Indian patients present with advanced gastric cancer and have a poor performance status, which makes their tolerability to chemotherapy poor. A phase 2 study from AIIMS, New Delhi, evaluated low-dose cisplatinum (15 mg/m2), etoposide (40 mg/m2), and paclitaxel (50 mg/m2), CEP, chemotherapy given on day 1 and 4 every 3 weeks in 33 patients with advanced disease (15). Around 26 of 33 patients showed response to CEP chemotherapy (2 complete responses, 21 partial responses, and 3 stable diseases). Four patients were operable after CEP chemotherapy. The average age was 52 years; about two cycles of chemotherapy was given and the average survival & disease progression free phase was 10 and 8 months. The incidence of grade 3 and 4 toxicity was 35% (most common being neutropenia); overall the chemotherapy was well tolerated. This study suggests that patients with advanced disease can also benefit from combination low-dose chemotherapy. The role of NACT has also been explored in Indian patients with inoperable tumor. In a study from AIIMS, New Delhi, 10 patients with locally advanced inoperable tumor received NACT with two cycles of cisplatin (30 mg/m2) and 5-fluorouracil (1,000 mg/m2). Eight of them showed objective response, six could undergo curative surgery, and the average survival was 10 months (range 1–60 months).
Combined modality therapy
A combination of chemotherapy and radiotherapy has been very effective in certain malignancies like head and neck cancers and anorectal cancers. The chemotherapy potentiates the effect of radiation therapy and helps in controlling distant metastasis. Preoperative chemoradiotherapy looks attractive as it has the potential to downsize tumors and make inoperable gastric cancer operable. In a phase 3 study by Stahl et al., patients with unresectable gastric cancer were randomized to NACT and surgery or induction chemotherapy followed by neoadjuvant chemo-radiotherapy and surgery (16). Patients in the chemo-radiotherapy group had a statistically significant higher pathological complete response rate (15.6% vs. 2%), with the overall survival at 3 years showing a trend toward better prognosis with chemo-radiotherapy (47.4% vs. 27.7%). Even in patients with resectable gastric cancer, preoperative chemo-radiotherapy improves pathological complete response rates and increases the success of D2 lymphadenectomy. Postoperative chemo-radiotherapy was evaluated in the SWOG9008/INT-0116 trial, now famously referred as the Macdonald trial. The trial included 556 patients with adenocarcinoma of stomach and lower gastroesophageal junction, stage 1–4 (nonmetastatic) operated tumors. Patients were randomized to only surgery or surgery with adjuvant chemo-radiotherapy. The chemo-radiotherapy protocol included 5-flurouracil and folinic acid (bolus regime) given in a schedule of one cycle before radiotherapy, two cycles concurrent with radiation, and two cycles after the completion of radiotherapy. Patients in the chemo-radiotherapy arm had a better overall survival (50% vs. 41%, P=0.05), 3-year relapse-free survival (48% vs. 31%), and lesser local recurrence (19% vs. 29%). This study showed that the chemo-radiotherapy can be effective as adjuvant treatment in gastric cancer. However, the Macdonald trial has faced several criticisms some of which include inadequate surgery as only 10% of patients underwent D2 lymphadenectomy, and 54% underwent a less extensive D1 dissection. Critics argue that a more extensive surgery would have probably negated the advantage obtained from chemo-radiotherapy.
There are no published data on radiotherapy or chemo-radiotherapy in gastric cancers from India and all our practice has been based on findings of the Western literature. There is an urgent need for well-designed RCTs on chemotherapy and chemo-radiotherapy from the Indian subcontinent, as we believe that the biology of gastric cancer from the subcontinent differs from the rest of the world. Extrapolating data from the rest of the world might not serve the best interests of our patients.
Targeted therapy
Targeted therapy including monoclonal antibodies and tyrosine kinase inhibitors have shown efficacy in various solid tumors in breast, lung, and colon cancer. The expression of HER2 gastric lesions reveals poor prognosis. HER2 function is blocked by the monoclonal antibody trastuzumab and the efficacy of trastuzumab is well proved in HER2 related breast malignancies. The TOGA study was a phase 3 RCT, which evaluated trastuzumab along with cisplatin and 5-flurouracil therapy in 594 patients with HER2-positive advanced gastric cancer. Trastuzumab addition to chemotherapy improved the overall survival significantly (13.5 vs. 11.5 months). There were no increased adverse effects with trastuzumab therapy (17).
Conclusions
The gastric cancer incidence is decreasing in developing nations and more proximal cancers are reported. However, in India the major population-based cancer registries report an incidence decline only in Mumbai and Chennai. A shift from distal to proximal as the site of disease has not been reported from India. Though the AAR of gastric cancer is low in majority of PBCR (population based cancer registry), the absolute number is still high because of the size of India’s population. The presentation of gastric cancer is usually advanced in Indian scenario due to deficiency in endoscopy services relative to patients with upper gastro intestinal symptoms.
Surgery forms the main stay of treatment in many gastric cancer patients. Neo adjuvant chemotherapy followed by surgery is being adopted in tertiary care hospitals with access to Multi-Disciplinary Team management of gastric cancer. However, when we search for the contribution of the Indian scientific fraternity to the world medical literature for gastric cancer, it is clear that a lot more is to be done; the possible reason may be a busy clinical schedule or lack of initiatives. Clinicians need to take out time from their busy clinical schedule and devote more time to research for the larger benefit to the society. There is an urgent need for research in various aspects of gastric cancer from India including epidemiological and therapeutic areas.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors haves no conflicts of interest to declare.
References
- Pavithran K, Doval DC, Pandey KK. Gastric cancer in India. Gastric Cancer 2002;5:240-3. [Crossref] [PubMed]
- Saha AK, Maitra S, Hazra SC. Epidemiology of Gastric Cancer in the Gangetic Areas of West Bengal. ISRN Gastroenterol 2013. Available online: https://www.hindawi.com/journals/isrn/2013/823483/
- Malhotra SL. Geographical distribution of gastrointestinal cancers in India with special reference to causation. Gut 1967;8:361-72. [Crossref] [PubMed]
- Ponnala D, Madireddi S. Evaluation of risk factors for gastric cancer. Int J Appl Biol Pharm Technol 2010;1:158-61..
- Indian Council of Medical Research (ICMR). First Report of the Population Based Cancer Registries Under North Eastern Regional Cancer Registry 2003-2004. Available online: http://www.icmr.nic.in/ncrp/first_report_2003-04/Starting%20Pages.pdf
- Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 2003;14 Suppl 2:ii31-6. [Crossref] [PubMed]
- Perrin D, Ruskin HJ, Niwa T. Cell type-dependent, infection-induced, aberrant DNA methylation in gastric cancer. J Theor Biol 2010;264:570-7. [Crossref] [PubMed]
- Park B, Shin A, Park SK, et al. Ecological study for refrigerator use, salt, vegetable, and fruit intakes, and gastric cancer. Cancer Causes Control 2011;22:1497-502. [Crossref] [PubMed]
- Choi BH, Song HS, An YS, et al. Relation Between Fluorodeoxyglucose Uptake and Glucose Transporter-1 Expression in Gastric Signet Ring Cell Carcinoma. Nucl Med Mol Imaging 2011;45:30-5. [Crossref] [PubMed]
- Kriplani AK, Kapur BM. Laparoscopy for pre-operative staging and assessment of operability in gastric carcinoma. Gastrointest Endosc 1991;37:441-3. [Crossref] [PubMed]
- D’souza MA, Singh K, Shrikhande SV. Surgery for gastric cancer: an evidence-based perspective. J Cancer Res Ther 2009;5:225-31. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- Shrikhande SV, Shukla PJ, Qureshi S, et al. D2 lymphadenectomy for gastric cancer in Tata Memorial Hospital: Indian data can now be incorporated in future international trials. Dig Surg 2006;23:192-7. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Sharma A, Raina V, Lokeshwar N, et al. Phase II study of cisplatin, etoposide and paclitaxel in locally advanced or metastatic adenocarcinoma of gastric/gastroesophageal junction. Indian J Cancer 2006;43:16-9. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6.9.
- Sharma A, Radhakrishnan V. Gastric cancer in India. Indian J Med Paediatr Oncol 2011;32:12-6. [Crossref] [PubMed]
Cite this article as: Ibrahim M, Gilbert K. Management of gastric cancer in Indian population. Transl Gastroenterol Hepatol 2017;2:64.