
Successful treatment of liver and abdomen metastasis of renal epithelioid angiomyolipoma with apatinib: a case report
Introduction
Angiomyolipoma (AML) is a histologically complex mesenchymal tumor with thick-walled blood vessels, adipose tissue and smooth muscle cells. The kidney remains the most commonly involved site. Renal epithelioid AML (EAML) belongs to the perivascular epithelioid cell tumor family, which is predominantly composed of epithelioid cells, with minimal or no adipose tissue. EAML is a rare tumor with malignant potential, affecting less than one patient per 10,000 populations. Unfortunately, metastatic EAML has a poor prognosis due to the lack of effective drugs. Here, we describe a rare case of a 53-year-old man with left renal EAML with multiple metastases in the greater omentum and liver. The patient successively experienced surgical resection, arterial chemoembolization, liver lesion aspiration, and cryoablation with an argon-helium knife, but he still experienced disease progression. As we know, angiogenesis contributes to tumor growth and metastasis, and antiangiogenic therapies remain an attractive tumor treatment method with demonstrated efficacy in treating a variety of solid tumors (1,2). Apatinib is a novel oral small-molecule tyrosine kinase inhibitor (TKI) that targets the intracellular domain of vascular endothelial growth factor receptor-2 (VEGFR-2) (3). Here, we present a case of advanced EAML with partial response to apatinib. To the best of our knowledge, this case is the first report of apatinib being used to treat EAML.
Case presentation
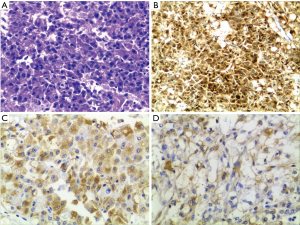
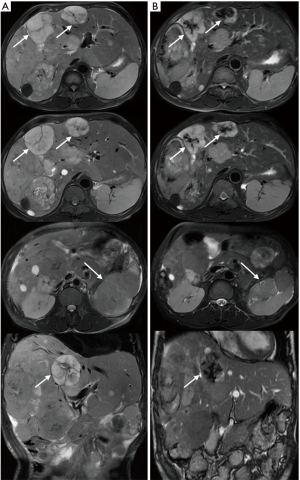
The patient was a 53-year-old man who underwent the left kidney and left renal neoplasm (10 cm × 8 cm × 6 cm) resection in 2002. A pathological examination showed EAML (Figure 1). Immunohistochemistry showed HMB45+++, Melan-A+, actin(SM)+, CKL−, VIM+/−, CgA+(partial), SYN+(partial), CD10+, and CD34+, which confirmed a pathological diagnosis of vascular endothelial component. However, in September 2009, the patient was hospitalized again for intestinal obstruction, and abdominal computed tomography (CT) showed that the ileum was compressed by multiple neoplasms at the greater omentum. Then, the patient underwent tumor resection, and pathological examination also showed EAML. The patient did not receive any post-surgical treatment. Unfortunately, in May 2011, a new neoplasm was found in the liver, with the same biopsy pathology. The patient then underwent a variety of treatments, including hepatic arterial chemoembolization, liver lesion aspiration, and cryoablation with an argon-helium knife. Despite these aggressive treatments, the liver lesion still progressed, and multiple enterocoelia lesions were found in 2014, the largest measuring 17.5 cm × 13.0 cm × 10.0 cm. Again, the patient underwent hepatic arterial chemoembolization and abdominal neoplasm resection. The postoperative pathological findings are the same as those previously described. Immunohistochemistry showed HMB45+, CKL−, VIM+, EMA−, CK7−, S-100+/−, desmin−, actin−, CD10+, and CD34+. In October 2015, the patient developed a series of symptoms, including abdominal distension, anorexia, chest congestion and shortness of breath. When his indigestion symptoms worsened, he consumed a liquid-only diet, which was simultaneously accompanied by intermittent fever in December 2015. On January 5, 2016, he was hospitalized for further treatment. The main symptoms at the time of admission were abdominal distension, firm and hepatomegaly, with the lower edge 5 cm under the right costal margin. The MR images from January 8, 2016 showed multiple nodular hypointense lesions on T1-weighted images, and on T2-weighted images, hyperintense lesions were observed in the liver parenchyma. The two largest lesions were measured approximately 6.0 cm × 5.5 cm and 5.6 cm × 3.8 cm on transverse images, and they appeared hyperintense on DW images. In the left kidney area, there were multiple abnormal nodular signals. The largest transverse lesion was measured approximately 8.1 cm × 5.8 cm; it was hypointense on T1-weighted images, hyperintense on T2-weighted images and apparently hyperintense on DW images. There were no obvious abnormal signs on the chest CT. Because of the patient’s extensive disease, poor condition and no chemotherapy reported can prolong survival (4), we decided to try targeted therapy instead of chemotherapy. After providing signed informed consent, the patient was administered apatinib (Hengrui Pharmaceutical Co., Ltd., Shanghai, China) at a dose of 500 mg/day on January 10, 2016. After 2 weeks of treatment, his appetite and abdominal distension improved, but he demonstrated apparent level III side effects, including multiple skin rash and oral ulcers, thus, the dosage was reduced by half. Then, he continued with a maintenance dose of apatinib 250 mg/day, which was well tolerated. It has been 7 months since the apatinib treatment, and the symptoms mentioned above have nearly disappeared. Follow-up MRI re-examinations showed that the size of the two largest lesions in the liver are about 5.5 cm × 4.8 cm and 4.5 cm × 3.1 cm. The abnormal signals in left kidney fossa are also smaller than before, the largest one is about 8.9 cm × 3.7 cm on transverse (Figure 2). As shown in Figure 2, it was marked by central necrosis in the lesions which is encouraging (as indicated by the arrows). However, a curative effect evaluation determined that the patient had not yet reached partial response (PR). A progression-free survival time of more than 8 months has been achieved, and the patient is now still undergoing the apatinib treatment, without any major toxic effects.
Discussion
AML is divided into typical renal AML and EAML. EAML, originating from mesenchymal neoplasm and primarily composed of epithelioid cells, may possess the characteristic of potentially malignance (5). Renal EAML is a rare tumor, the morbidity is less than one patient per 10,000 populations. The number of cases reported in the literature is relatively small. A Cleveland Clinic group has reported only 16 renal epithelioid AMLs after reviewing 209 AMLs surgically treated during a 26-year period. Studies have reported that some EAML cases had poor prognoses because of the high incidence of local recurrence, invasive growth, venous invasion, lymph nodes or distant metastasis (particularly liver and lung) (6,7). In previous reports, the disease progression was relatively rapid, and many cases resulted in death within 2 years after recurrence or metastasis (8-10). However, there are also case reports about EAML patients with pulmonary metastasis with slowly progressing disease (11).
The first choice for primary and metastatic lesions is surgical resection or radiotherapy but only when impossible, and no effective therapy has been established for advanced cases. A few studies have been performed in this field, and most are case reports due to the rarity of the disease. These case reports have primarily included chemotherapy and targeted drug therapy. The most common chemotherapeutic drugs include doxorubicin, cyclophosphamide, and cisplatin. However; the relatively high efficacy of these therapies has not translated to improved survival rates (4). Bissler et al. (12) has reported that the inhibitor of the mammalian target of rapamycin (mTORi), such as everolimus, could reduce tuberous sclerosis-related AML volume. In addition, some recent reports (13-15) have shown that everolimus has promising therapeutic effects on invasive EAML. However, these studies are all case reports, and most have short effective times; long-term validity has not yet been observed. Targeted therapies that inhibit the micro vascularization of tumors have demonstrated therapeutic effects and tolerable adverse reactions. Note that such tumors have rich vascular supplies, and immunohistochemistry often shows a positive expression of CD34, which indicates that anti-angiogenesis drugs may be effective. Apatinib (16), a small-molecule inhibitor of VEGFR-2, is an orally bioavailable agent currently being studied in multiple tumor types as a third- or fourth-line treatment for patients with advanced gastric cancer, and it is being studied in phase II and III clinical trials in non-small cell lung cancer, breast cancer, gastric cancer, hepatocellular carcinoma and colorectal cancer (17). However, it has not yet been reported in EAML. In this case, an EAML patient with multiple liver and abdomen metastases refused to accept any toxic chemotherapy, then apatinib monotherapy was used to control the disease. After 8 months of maintenance treatment, the volume of the liver and enterocoelia metastatic lesions decreased, and the ascites disappeared. To date, there has been no effective treatment for metastatic EAML. This case indicated that apatinib is effective in treating metastatic EAML, and it is worthy of further clinical trials.
Conclusions
Here, we described a rare case of a patient with left renal EAML with multiple metastases who underwent several failed treatments but showed a partial response to apatinib. Therefore, we speculate that apatinib may provide an additional treatment option for EAML, particularly for patients in poor condition.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.41). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peng Y, Cui H, Liu Z, et al. Apatinib to combat EGFR-TKI resistance in an advanced non-small cell lung cancer patient with unknown EGFR status: a case report. Onco Targets Ther 2017;10:2289-95. [Crossref] [PubMed]
- Peng QX, Han YW, Zhang YL, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 2017;8:52813-22. [PubMed]
- Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005;209-31. [Crossref] [PubMed]
- Cibas ES, Goss GA, Kulke MH, et al. Malignant epithelioid angiomyolipoma ('sarcoma ex angiomyolipoma') of the kidney: a case report and review of the literature. Am J Surg Pathol 2001;25:121-6. [Crossref] [PubMed]
- Bakshi SS, Vishal K, Kalia V, et al. Aggressive renal angiomyolipoma extending into the renal vein and inferior vena cava - an uncommon entity. Br J Radiol 2011;84:e166-8. [Crossref] [PubMed]
- Lopater J, Daniel L, Akiki A, et al. Renal epithelioid angiomyolipoma. Prog Urol 2009;19:457-61. [Crossref] [PubMed]
- Compérat EV, Vasiliu V, Ferlicot S, et al. Tumors of the kidneys: new entities. Ann Pathol 2005;25:117-33. [PubMed]
- Sato K, Ueda Y, Tachibana H, et al. Malignant epithelioid angiomyolipoma of the kidney in a patient with tuberous sclerosis: an autopsy case report with p53 gene mutation analysis. Pathol Res Pract 2008;204:771-7. [Crossref] [PubMed]
- Nese N, Martignoni G, Fletcher CD, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol 2011;35:161-76. [Crossref] [PubMed]
- Brimo F, Robinson B, Guo C, et al. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol 2010;34:715-22. [PubMed]
- Shigenobu T, Kohno M, Emoto K, et al. A solitary metastatic lung tumor slow-growing with late onset from renal epithelioid angiomyolipoma. Ann Thorac Cardiovasc Surg 2014;20:445-8. [Crossref] [PubMed]
- Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013;381:817-24. [Crossref] [PubMed]
- Faria E, Turturro F, Rao P, et al. Malignant epithelioid angiomyolipoma: tumor and metabolic response to everolimus as evaluated with positron emission tomography. Clin Genitourin Cancer 2013;11:e1-5. [Crossref] [PubMed]
- Shitara K, Yatabe Y, Mizota A, et al. Dramatic tumor response to everolimus for malignant epithelioid angiomyolipoma. Jpn J Clin Oncol 2011;41:814-6. [Crossref] [PubMed]
- Kohno J, Matsui Y, Yamasaki T, et al. Role of mammalian target of rapamycin inhibitor in the treatment of metastatic epithelioid angiomyolipoma: a case report. Int J Urol 2013;20:938-41. [Crossref] [PubMed]
- Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc) 2015;51:223-9. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]


