
Co-targeting Aurora kinase A and Bcl-2 synergistically inhibits the viability in double-hit lymphoma cells
Introduction
Double-hit lymphoma (DHL) has been defined as high grade B cell lymphomas with MYC and Bcl-2 or Bcl-6 translocations according to the 2016 World Health Organization classification (1). DHL has been becoming the focus of intense investigation due to its intractable clinical behaviors and dismal prognoses. Most DHL cases possess concomitant translocations of MYC and Bcl-2 (58–87%), but few harbor MYC/Bcl-6 dual rearrangements (2-5). DHL patients tend to have a plurality of unfavorable prognostic parameters, including high tumor burden, elevated serum lactate dehydrogenase levels, advanced stage, high International Prognostic Index score, and extranodal involvement (6). The conventional immunochemotherapy regimen (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and aggressive therapeutic regimens (R-DA-EPOCH, R-Hyper CVAD and R-CODOX-M/IVAV) did not remarkably improve the patient outcomes, especially overall survival (OS), with median OS of no more than 2 years (7,8). A majority of patients succumb to refractory or relapsed diseases after a short remission.
The main characteristics of DHL are the translocations of MYC and Bcl-2, and their dysfunction has indeed been confirmed to contribute to malignant cell growth and pathogenesis in DHL (6,9,10). One of the alternative strategies is the combination treatment of two promising inhibitors of MYC and Bcl-2 for DHL patients (11,12). MYC directly regulates Aurora kinase A (Aurka), which in turn expedites MYC transcription by binding to its promoter, thus forming a feedback loop between MYC and Aurka (13,14). Aurka is a member of highly conserved serine/threonine Aurora kinase family, and participates in the regulation of multiple cell cycle aspects, particularly in the G2/M phase. Aurka is highly elevated in aggressive lymphomas, such as MYC-driven B-cell lymphomas and other types of lymphoma with high growth fractions, and it is also correlated with disease activity and prognosis (13,15,16). Alisertib (MLN8237), a highly specific second-generation Aurka inhibitor, has been demonstrated to have some encouraging preclinical activities and been underway in clinical trials for the treatment of several tumors (17). Bcl-2 regulates the intrinsic apoptosis pathway and therefore stimulates chemoresistance (18). Strategies that antagonize Bcl-2 function have been widely developed due to the emergence of a number of promising BH3 mimetics. ABT-199 (Venetoclax), a selective third-generation BH3 mimetic, has gained FDA approval as a monotherapy for chronic lymphocytic leukemia (19). Studies have reported the synergistic antitumor effects between ABT-199 and other agents, suggesting that the potential value of the combination therapy between ABT-199 and other novel inhibitors may be observed in some highly invasive tumors (20,21). Moreover, ABT-199 alone has also exerted preclinical efficacy in murine MYC-driven lymphoma models (22).
Herein, the purpose of this study was to evaluate the combination treatment of alisertib and ABT-199 in DHL cells. This was the first time to demonstrate that synergistic effects of Aurka inhibitors and Bcl-2 inhibitors, which was potentially therapeutic strategies for DHL lymphoma.
Methods
Cells and reagents
The DHL cell lines used in this study (DOHH2 and VAL) were provided by Dr. Kai Fu (University of Nebraska Medical Center, NE, USA), which had been validated by STR DNA finger printing (Figures S1,S2). DOHH2 was maintained in RPMI-1640 medium supplemented with 15% fetal bovine serum (FBS, Thermo Fisher Scientific, MA, USA). VAL was cultured in Iscove’s modified Dulbecco’s medium (IMDM, GE Healthcare, Illinois, USA) containing 10% FBS. All media were supplemented with 1% penicillin/streptomycin, and cells were incubated under 37 °C humid condition with 5% CO2. Alisertib and Nocodazole (Noc) were purchased from Selleck Chemicals (Houston, TX, USA). ABT-199 was supplied by MedChem Express (NJ, USA).
Cell proliferation assay
DHL cells were treated with ABT-199 or alisertib at concentrations ranging from 10 to 10,000 nM for 24, 48 and 72 h. Changes in cell viability were evaluated using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, USA, Cat.#G3580) following the manufacturer’s protocol. The 50% inhibitory concentration (IC50) values were calculated utilizing GraphPad Prism 5.0 software.
Drug combinations and synergy evaluation
Cells were incubated with single or combination treatment of alisertib and ABT-199 at concentrations of 1/5–5/5 IC50 for 48 h. MTS assay was then performed as described above. The effects of the combination treatment were calculated by the CompuSyn software using the Chou-Talalay combination index (CI) and isobologram methods according to the median-effect principle (23). The combination effect was designated synergism (CI <1.0), addition (CI =1.0), or antagonism (CI >1.0).
Cell cycle assay
DHL cells were treated with ABT-199 or/and alisertib at 2/5 IC50 for 48 h, then washed and resuspended in PBS, and fixed by dropwise addition of ice-cold absolute ethanol to a final concentration of 70%. Fixed cells were stored at 4 °C overnight, pelleted and incubated with RNase A (Solarbio, China, final concentration was 0.2 mg/ml), and propidium iodide (PI, Solarbio, China, final concentration was 50 µg/mL) for 30 min at 37 °C protected from light. Cell cycle was analyzed on a FACSCelesta flow cytometer (BD Bioscience, CA, USA) by ModFit LT 4.1 software.
Apoptosis assay
Cells were treated with ABT-199 or/and ALS at 3/5 IC50 for 48 h, then were rinsed with cold PBS once. After centrifugation at 300 ×g for 5 min, cells were resuspended in 200 µL of binding buffer (BD Bioscience, CA, USA), and then 3 µL of Annexin V-FITC and 3 µL of PI were added. The samples were analyzed by FACSCelesta post incubation for 15 min in the dark at room temperature (RT). Results were analyzed with FlowJo V10 software.
Western blot analysis
Treated cells were harvested in RIPA lysis buffer with 1 mM PMSF and phosphatase inhibitor cocktail II. The cell lysates were centrifuged at 12,000 ×g at 4 °C for 10 min. The protein concentration was measured using the BCA method (Thermo Fisher Scientifc, MA, USA). A 30 µg of total protein was separated by 10% SDS-PAGE gel electrophoresis and subsequently transferred to PVDF membranes (Merck Millipore, Darmstadt, Germany) at 260 mA for 2 h. Then, the blot was blocked in 5% bovine serum albumin for 1 h at RT and incubated with the specific primary antibodies at 4 °C overnight. After washing in tris-buffered saline–Tween thrice for 10 min, the blot was probed with 1:5,000 dilution of goat anti-rabbit IgG or anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich, MO, USA) for 1h at RT. Signals were detected using a chemiluminescence reagent Immobilon (Merck Millipore, Darmstadt, Germany). Signal intensity was obtained by Image Studio Lite software. For the detection of Aurka phosphorylation, the cells were first synchronized by 200 ng/mL Noc for 10 h, then were untreated or treated with alisertib or/and ABT-199 for 1 h. Antibodies used, including rabbit polyclonal anti-MYC (1:1,000, Cat.#10828-1-AP), rabbit polyclonal anti-Bcl2 (1:2,000, Cat.#12789-1-AP), rabbit polyclonal anti-p53(1:1,000, Cat.#10442-1-AP), mouse monoclonal anti-p21(1:10,00, Cat.# 60214-1-Ig) and rabbit polyclonal anti-MCL1(1:1000, Cat.#16225-1-AP) were purchased from ProteinTech Group, Inc. (Hubei, China). Rabbit monoclonal anti-phospho-Aurka (Th288, 1:1,000, Cat.#3079), rabbit monoclonal anti-Aurka (1G4, 1:1,000, Cat.#4718), mouse monoclonal anti-Caspase-3 (1:1,000, Cat.#9668), mouse monoclonal anti-cleaved PARP (1:1,000, Cat.#9546), mouse monoclonal anti-CDK1/cdc2 (1:1,000, Cat.#9116), and rabbit monoclonal anti-Cyclin B1(1:1,000, Cat.#12231) were obtained from Cell Signaling Technology (MA, USA). Mouse monoclonal anti-β-actin (1:2,000, clone AC-15, A1978) was supplied by Sigma-Aldrich and used as an endogenous protein for normalization.
Statistical analysis
All assays were performed three times, and data were displayed as the mean ± standard deviation (SD). Differences were analyzed by one-way ANOVA, using IBM SPSS Statistics 20 software. P<0.05 was considered significant.
Results
ABT-199 and alisertib inhibited the DHL cell proliferation
Cell proliferation was examined in DHL cells after exposure to ABT-199 or alisertib. Figure 1 showed that ABT-199 and alisertib suppressed the cell viability in a dose- and time-dependent manner in DOHH2 and VAL cells, respectively. The IC50 values of the two drugs ranged from 15 nM to over 1,000 nM for 24, 48 and 72 h (Table 1), suggesting that ABT-199 and alisertib inhibited the cell proliferation in DHL cells.
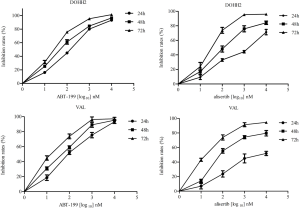

Full table
ABT-199 combined with alisertib synergistically inhibited the DHL cell proliferation
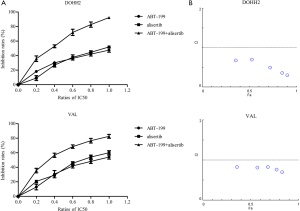
DOHH2 and VAL cells were exposed to alisertib combined with ABT-199 at a panel of concentrations (1/5–5/5 IC50). Figure 2A revealed that the combination treatment promoted stronger inhibitory activity and synergistically suppressed the DHL cell growth compared to either agent alone. Median dose-effect analysis of the combination treatment suggested that ABT-199 and alisertib had synergistic cytotoxic effects (CI <1.0) in DHL cells (Figure 2B).
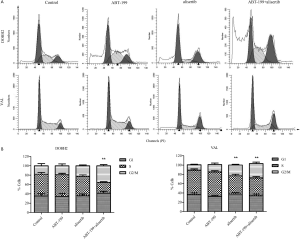
ABT-199 combined with alisertib induced G2/M phase arrest in DHL cells
Cell cycle analyses were further performed to characterize the antitumor efficacy of the combination treatment in DOHH2 and VAL cells. As shown in Figure S3, the combined treatment tended to induce more cell accumulation in G2/M phase. These results suggested that alisertib combined with ABT-199 seemed to boost G2/M phase arrest in DHL cells.
ABT-199 and alisertib regulated p53/p21 signaling in DOHH2 cells
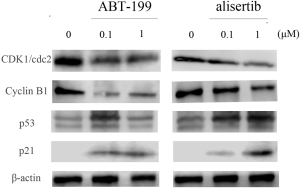
To study the mechanisms of inducing G2/M phase arrest, we first analyzed the expression of key cell-cycle regulators, including CDK1/cdc2 and cyclin B1, in DOHH2 cells after treatment with ABT-199 and alisertib at 0.1 and 1 µM for 24 h. Figure 3 displayed that the expression of CDK1/cdc2 and cyclin B1 was significantly reduced by alisertib. Strikingly, we also observed that ABT-199 could downregulate the expression of CDK1/cdc2 and cyclin B1. To further investigate the underlying upstream mechanism to regulate the CDK1/cdc2 and cyclin B1, we next detected the expression of p53 and p21. We found that the expression of p53 and p21 were upregulated by both alisertib and ABT-199, suggesting that the two agents regulated p53/p21 signaling in DOHH2 cells.
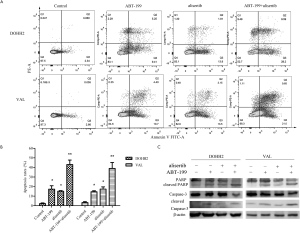
ABT-199 combined with alisertib synergistically induced intrinsic apoptosis in DHL cells
Apoptosis assays were performed to determine whether cell apoptosis was engaged in the cytotoxicity of alisertib and ABT-199 in DHL cells. As shown in Figure S4A,B, the combination treatment induced a consistently significant increase in the percentages of apoptotic cells compared to single-agent treatment (P<0.05). We then examined whether the cell intrinsic apoptotic pathway was regulated by alisertib and ABT-199. Figure S4C revealed that the combination treatment elevated the expression of cleaved caspase-3 and PARP, which was not evidently observed in DHL cells after single-agent treatment. In addition, due to that MCL1 expression had been reported to be a synergistically apoptotic mechanism during the combination treatment between ABT-199 and other drugs (24), MCL1 expression was also detected after exposure to alisertib. Our results showed that alisertib did not downregulate the MCL1 expression (Figure S5). Meanwhile, the combination treatment also triggered cell morphologic changes in accordance with an apoptotic phenotype characterized by cell shrinkage, chromatin condensation, and membrane bubbling in DHL cells under light microscopy (data not shown).
ABT-199 combined with alisertib enhanced the inhibition of targeted protein expression
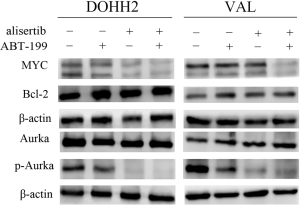
The drug-targeted protein levels were further assessed to understand the mechanisms of alisertib and ABT-199 in DHL cells. Figure 4 showed that there was a remarkable decrease in MYC expression upon combination treatment. Unexpectedly, changes of Bcl-2 expression were not observed in DOHH2 and VAL cells. Some studies had shown that auto-phosphorylation of Aurka was a primary requisite for kinase activity on T288 site (25-27). Therefore we evaluated the alterations of p-Aurka on T288 site in DHL cells. Results showed that the levels of p-Aurka were slightly downregulated in cells treated with ABT-199, and were obviously decreased in cells treated with alisertib. The combination treatment further amplified the effects.
Discussion
So far, the standard of care is lacking for DHL. Intensified chemotherapy including R-DA-EPOCH is the preferred treatment protocol in multiple cancer centers. R-DA-EPOCH improved the progress-free survival (PFS) without OS benefit for DHL patients (28,29). Thus, novel alternative treatment modalities should be investigated to improve the prognosis of these high-risk patients (30). Targeted therapies, such as MYC and Bcl-2 based on the underlying genetic lesions of DHL, have been comprehensively explored due to their oncogenic cooperation (31). Preclinical data also suggested that targeting single oncogene (MYC or Bcl-2) might be inadequate for this lymphoma subtype (32).
MYC is an essential transcription factor which modulates diverse cellular functions, including cell proliferation, cell cycle, migration, differentiation, and metabolism (33,34). Although pharmacological attempts to directly inhibit oncogenic MYC have not borne fruit (35), the improved appreciation of MYC regulation has led to the continuous development of a range of therapeutic approaches targeting MYC-related tumors (36,37). In current study, alisertib was selected for the combination treatment owing to the intimate crosstalk between MYC and Aurka. Aurora kinase is essential for the tumor maintenance of MYC-associated lymphoma (12), whereas blocking Aurora enzyme activity stimulates apoptosis in a MYC-driven lymphoma murine model (13). Thus, simultaneous inhibition of Aurka and Bcl-2 may be required for optimal anti-lymphoma activity in DHL.
Aurka inhibitors have displayed synergistic effects in combination treatment with vincristine and rituximab for hematological malignancies (38,39), and alisertib has also exhibited preliminary clinical efficacy in relapsed/refractory non-Hodgkin lymphoma (40). In our study, the combination treatment with ABT-199 and alisertib exerted synergistic activity in the inhibition of cell viability, and induction of G2/M phase arrest and cell apoptosis in DHL cells. In particular, the combination treatment induced an enhanced delay in G2/M transition at low concentrations, followed by inducing significant cell apoptosis at higher concentrations. Studies have shown that inactivating Aurka activity leaded to increased stability of p53 and G2/M arrest in other tumor cells (41,42), which was consistent with our data in DHL cells. However, the role of ABT-199 has been poorly clarified in cell cycle regulation. In this study, we identified for the first time that ABT-199 could boost G2/M phase arrest via modulating the expression of CDK1/cdc2, cyclin B1, p21 and p53.
Taken together, our study showed that co-targeting Aurka and Bcl-2 could represent a novel alternative treatment strategy for DHL. Hence, this combination treatment between alisertib and ABT-199 should be subjected to future clinical trials to overcome specific molecular vulnerabilities in DHL lymphoma.



Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (81402945), Scientific Research Foundation for the Returned Overseas Chinese Scholars in Tianjin (2015-31), Key projects of Tianjin Municipal Health Bureau (15KG145).
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.41). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013;122:3884-91. [Crossref] [PubMed]
- Li SY, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18) (q32;q21): an aggressive disease with heterogeneous histology, germina center B-cell immunophenotype and poor outcome. Mod Pathol 2012;25:145-56. [Crossref] [PubMed]
- Pillai RK, Sathanoori M, Van Oss SB, et al. Double-hit B-cell Lymphomas With BCL6 and MYC Translocations Are Aggressive, Frequently Extranodal Lymphomas Distinct From BCL2 Double-hit B-cell Lymphomas. Am J Surg Pathol 2013;37:323-32. [Crossref] [PubMed]
- Lin P, Medeiros LJ. The Impact of MYC Rearrangements and "Double Hit" Abnormalities in Diffuse Large B-Cell Lymphoma. Curr Hematol Malig Rep 2013;8:243-52. [Crossref] [PubMed]
- Li SY, Lin P, Young KH, et al. MYC/BCL2 Double-Hit High-Grade B-Cell Lymphoma. Adv Anat Pathol 2013;20:315-26. [Crossref] [PubMed]
- Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 2009;114:2273-9. [Crossref] [PubMed]
- Cheah CY, Oki Y, Westin JR, et al. A clinician's guide to double hit lymphomas. Br J Haematol 2015;168:784-95. [Crossref] [PubMed]
- Leskov I, Pallasch CP, Drake A, et al. Rapid generation of human B-cell lymphomas via combined expression of Myc and Bc12 and their use as a preclinical model for biological therapies. Oncogene 2013;32:1066-72. [Crossref] [PubMed]
- Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica 2007;92:1297-301. [Crossref] [PubMed]
- Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev 2017;31:37-42. [Crossref] [PubMed]
- Nowakowski GS, Czuczman MS. ABC, GCB, and Double-Hit Diffuse Large B-Cell Lymphoma: Does Subtype Make a Difference in Therapy Selection? Am Soc Clin Oncol Educ Book 2015;e449-57. [Crossref] [PubMed]
- den Hollander J, Rimpi S, Doherty JR, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 2010;116:1498-505. [Crossref] [PubMed]
- Lu LF, Han H, Tian Y, et al. Aurora kinase A mediates c-Myc's oncogenic effects in hepatocellular carcinoma. Mol Carcinog 2015;54:1467-79. [Crossref] [PubMed]
- Farag SS. The potential role of Aurora kinase inhibitors in haematological malignancies. Br J Haematol 2011;155:561-79. [Crossref] [PubMed]
- Yakushijin Y, Hamada M, Yasukawa M. The expression of the Aurora-A gene and its significance with tumorgenesis in non-Hodgkin's lymphoma. Leuk Lymphoma 2004;45:1741-6. [Crossref] [PubMed]
- Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert Opin Drug Discov 2011;6:291-307. [Crossref] [PubMed]
- Kang MH, Reynolds CP. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin Cancer Res 2009;15:1126-32. [Crossref] [PubMed]
- Deeks ED. Venetoclax: First Global Approval. Drugs 2016;76:979-87. [Crossref] [PubMed]
- Johnson-Farley N, Veliz J, Bhagavathi S, et al. ABT-199, a BH3 mimetic that specifically targets Bcl-2, enhances the antitumor activity of chemotherapy, bortezomib and JQ1 in "double hit" lymphoma cells. Leuk Lymphoma 2015;56:2146-52. [Crossref] [PubMed]
- Leber B, Geng F, Kale J, et al. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Rev Mol Med 2010;12:19. [Crossref] [PubMed]
- Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 2013;121:2285-8. [Crossref] [PubMed]
- Chou TC. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res 2010;70:440-6. [Crossref] [PubMed]
- Li L, Pongtornpipat P, Tiutan T, et al. Synergistic induction of apoptosis in high-risk DLBCL by BCL2 inhibition with ABT-199 combined with pharmacologic loss of MCL1. Leukemia 2015;29:1702-12. [Crossref] [PubMed]
- Littlepage LE, Wu H, Andresson T, et al. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci U S A 2002;99:15440-5. [Crossref] [PubMed]
- Satinover DL, Leach CA, Stukenberg PT, et al. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci U S A 2004;101:8625-30. [Crossref] [PubMed]
- Ohashi S, Sakashita G, Ban R, et al. Phospho-regulation of human protein kinase Aurora-A: analysis using anti-phospho-Thr288 monoclonal antibodies. Oncogene 2006;25:7691-702. [Crossref] [PubMed]
- Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354-61. [Crossref] [PubMed]
- Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol 2015;170:504-14. [Crossref] [PubMed]
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891-901. [Crossref] [PubMed]
- Sasaki N, Kuroda J, Nagoshi H, et al. Bcl-2 is a better therapeutic target than c-Myc, but attacking both could be a more effective treatment strategy for B-cell lymphoma with concurrent Bcl-2 and c-Myc overexpression. Exp Hematol 2011;39:817-28. [Crossref] [PubMed]
- Marullo R, Rutherford SC, Leonard JP, et al. Therapeutic implication of concomitant chromosomal aberrations in patients with aggressive B-cell lymphomas. Cell Cycle 2016;15:2241-7. [Crossref] [PubMed]
- Nie Z, Hu G, Wei G, et al. c-Myc Is a Universal Amplifier of Expressed Genes in Lymphocytes and Embryonic Stem Cells. Cell 2012;151:68-79. [Crossref] [PubMed]
- Smith SM, Anastasi J, Cohen KS, et al. The impact of MYC expression in lymphoma biology: Beyond Burkitt lymphoma. Blood Cells Mol Dis 2010;45:317-23. [Crossref] [PubMed]
- Delmore JE, Issa GC, Lemieux ME, et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell 2011;146:904-17. [Crossref] [PubMed]
- Tu WB, Helander S, Pilstal R, et al. Myc and its interactors take shape. Biochim Biophys Acta 2015;1849:469-83. [Crossref] [PubMed]
- Sewastianik T, Prochorec-Sobieszek M, Chapuy B, et al. MYC deregulation in lymphoid tumors: molecular mechanisms, clinical consequences and therapeutic implications. Biochim Biophys Acta 2014;1846:457-67. [PubMed]
- Yoshida K, Nagai T, Ohmine K, et al. Vincristine potentiates the anti-proliferative effect of an aurora kinase inhibitor, VE-465, in myeloid leukemia cells. Biochem Pharmacol 2011;82:1884-90. [Crossref] [PubMed]
- Mahadevan D, Stejskal A, Cooke LS, et al. Aurora A Inhibitor (MLN8237) plus Vincristine plus Rituximab Is Synthetic Lethal and a Potential Curative Therapy in Aggressive B-cell Non-Hodgkin Lymphoma. Clin Cancer Res 2012;18:2210-9. [Crossref] [PubMed]
- Friedberg JW, Mahadevan D, Cebula E, et al. Phase II Study of Alisertib, a Selective Aurora A Kinase Inhibitor, in Relapsed and Refractory Aggressive B- and T-Cell Non-Hodgkin Lymphomas. J Clin Oncol 2014;32:44-U122. [Crossref] [PubMed]
- Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet 2004;36:55-62. [Crossref] [PubMed]
- Ding YH, Zhou ZW, Ha CF, et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther 2015;9:425-64. [PubMed]


