
Robotic assisted radical cystectomy versus open radical cystectomy: a review of what we do and don’t know
Introduction
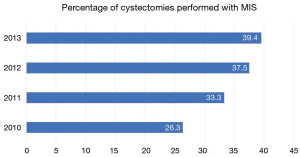
Radical cystectomy (RC) is the gold standard treatment for muscle-invasive and high-risk, noninvasive bladder cancer. Owing to its technical complexity and the relative frailty of patients often being considered for surgery, RC confers significant risks for morbidity and mortality. Traditionally, open radical cystectomy (ORC) has been the most common surgical approach. As in other disease states, robotic surgery has gained popularity following the first report of the robot-assisted radical cystectomy (RARC) by Menon et al. in 2003 (1). Even in the absence of level I evidence to support its superiority, RARC continues to gain ground on ORC (Figure 1). Not surprisingly, the increased availability and widespread dissemination of RARC has bred debate as to the optimal surgical approach. The technical benefits of minimally invasive surgery—notably, robotic platforms—have been well described. However, whether these putative advantages translate to improved patient outcomes or alleviate the overall cost burden of treatment remains to be proven. Herein we provide a contemporary critical review of the available data comparing ORC and RARC.
Methods
We performed a search of the PubMed, Cochrane Library, and Google Scholar databases during April 2019 to identify all relevant studies using the following keywords: “radical cystectomy” and “open versus robotic radical cystectomy.” Only comparative analyses between the open and robotic approaches were included in the review. Emphasis was placed on identifying prospective studies, including randomized controlled trials (RCTs), and meta-analyses that reported on perioperative, oncological, and functional outcomes. Cited references from the relevant studies were also assessed for potential inclusion. Case reports, single-center retrospective series, comments, editorials, letters, and articles not published in English were excluded. Four authors (ZGG, ABK, JSW, RM) independently screened the search results to select those studies most relevant to this review.
RCTs of RARC
In 2010, the first RCT comparing ORC and RARC was published by Nix et al. (3). The authors compared 21 RARC patients to 20 ORC patients who were operated on between 2008 to 2009. There were no significant differences in the baseline characteristics between the two groups. The primary endpoint, lymph node (LN) yield, was not significantly different between the two groups. However, RARC was associated with a significantly longer operative time (4.2 vs. 3.5 h; P<0.0001), a lower estimated blood loss (EBL) (258 vs. 500 cc; P<0.0001), lower narcotic requirements (89 vs. 147 mg of morphine equivalents; P=0.004), and a faster return of bowel function (3.2 vs. 4.3 days to first bowel movement; P=0.0008) (3).
A second RCT of comparable size, the Cystectomy Open Robotic and Laparoscopic (CORAL) trial, was published by Kahn et al. in 2016. It compared 20 RARC to 20 ORC patients who underwent surgery between 2009 to 2012. The primary endpoints were 30- and 90-day complications rates. They found no significant difference between major complications, defined as Clavien grade 3 or higher, at 30 or 90 days. Again, RARC was associated with a longer operative time (389 vs. 293 min; P<0.001), but a shorter length of stay (LOS) (12 vs. 14 days; P=0.031) and a shorter time to tolerating solids (4 vs. 7.5 days; P=0.03). There were no significant differences in EBL, time to flatus, or quality of life (QoL) (4).
In 2015, Bochner et al. published “Comparing Open Radical Cystectomy and Robot-Assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial” (NCT01076387). From 2010 to 2013, 118 patients were enrolled 60 of whom underwent RARC and 58 of whom underwent ORC (5,6). Once again, operative time was longer (456 vs. 329 min; P<0.001) but EBL was lower (516 vs. 676 cc; P=0.027) in the RARC group (6). There were no significant differences in Clavien 2–5 complications, LN yield, positive surgical margin (PSM) rate, LOS, or QoL at 3 and 6 months. A recent update of this trial with longer follow-up was published in 2018 (7). In that study, Bochner and colleagues evaluated long-term outcomes including recurrence free, cancer specific, and overall survival. The median follow-up was 4.9 years. There were no statistically significant differences in recurrence rates or cancer specific/overall survival. However, a large confidence interval made it difficult to render a definitive conclusion regarding recurrence rates. Interestingly, RARC patients had more peritoneal recurrences with abdominal wall involvement (n=5) compared to open cystectomy (n=2). It is important to note that comparing recurrence locations was not planned as part of the original analysis and that these values did not reach statistical significance.
The largest RCT to date is the RARC versus open radial cystectomy in patients with bladder cancer (RAZOR) trial (NCT01157676), which was a multi-institutional, non-inferiority trial that compared 176 RARC patients and 174 ORC patients who underwent surgery from 2011 to 2014 (8). With respect to the primary endpoint, which was disease progression, the authors found that RARC was non-inferior to ORC with a two-year follow up. Once again, operative time was significantly longer (428 vs. 361 min; P=0.0005) and EBL was significantly lower (300 vs. 700 cc; P<0.0001) in the RARC group. Likely secondary to a lower EBL, RARC was also associated with a lower incidence of intraoperative and postoperative transfusions (13% vs. 34%; P<0.0001 and 25% vs. 40%; P=0.0089, respectively). LOS was also significantly shorter in the RARC group (6 vs. 7 days; P=0.0216). There were no differences in the incidence of any or major complications at 90-days, LN yield, PSM rate, or 3- and 6-month QoL outcomes between the two groups. A comparison of the findings of these RCTs can be found in Table 1.

Full table
Recently, there have been two metanalyses of these RCTs, both of which also included an additional, 40-patient RCT (9). The first, by Satkunasivam et al., found no difference in recurrence free survival or progression free survival, PSM rate, or LN yield after RARC compared to ORC. Although recurrence rates were not significantly different between the two groups, patients who underwent RARC were more likely to have abdominal/distant recurrences as opposed to local recurrences. In addition, EBL was lower with RARC (difference ‒281 cc, 95% CI, ‒435 to ‒125) and mean operative time was shorter with ORC (difference 75 min, 95% CI, 26–123) (10). The second metanalysis, which did not include the Bochner 2018 data, found no difference in disease recurrence, 90-day incidence of major complications, and 90-day QoL among 458 patients. RARC was associated with a decreased incidence of perioperative transfusions (RR 0.58, 95% CI, 0.43–0.80) and shorter LOS (RR ‒0.63 days, 95% CI, ‒1.21 to ‒0.05 days). ORC was associated with shorter operative times (MD 68.51 min, 95% CI, 30.55–105.48 min). PSM rates were not significantly different between the two groups (11). These findings have been confirmed by another metanalysis performed on a subset of these RCTs (12).
Another systematic review and metanalysis that included RARC case series and prospective or retrospective comparisons of RARC to ORC found similar results (13). RARC was associated with less blood loss (WMD: −521; 95% CI, −644 to −399), lower incidence of transfusion (OR: 0.16; 95% CI, 0.1–0.27), shorter hospital stays (WMD: −1.26; 95% CI, −2.08 to −0.43), and longer operative times (WMD: 83.60; 95% CI, 57.1–110.1). RARC was also associated with a lower risk of complications at 90-days. There were no differences in complication rates at 30 days or 30- and 90-day mortality rates (13). Several other metanalyses, also found that RARC was associated with a lower EBL and transfusion rate, shorter hospital stays, longer operative times, greater LN yields, and similar incidences of PSMs (14-16). One of these studies found that RARC was associated with a significantly shorter time to flatus and lower 90-day complication rates (16).
Extracorporeal urinary diversion (ECUD) vs. intracorporeal urinary diversion (ICUD)
A criticism of the aforementioned RCTs is that all patients underwent ECUD, thereby nullifying some of the benefits of minimally invasive surgery. Potential advantages of ICUD include decreased fluid losses, reduced EBL, smaller incisions, reduced pain, and faster return of bowel function (17,18). Notably, the utilization of ICUD has risen dramatically in the last decade. Among members of the International Robotic Cystectomy Consortium (IRCC), from 2005 to 2016 the percentage of all diversions that were intracorporeal increased from 9% to 91% (18). Therefore, the generalizability of these studies is unknown.
Currently, there are only a few studies comparing outcomes after ECUD and ICUD. One recent retrospective analysis of ECUD (all of which were ileal conduits) and ICUD, found that total operative time (375 vs. 330 min; P=0.019), EBL (425 vs. 300 cc; P=0.035), and 30-day complications rates (71.4% vs. 48.4%; P=0.008) were lower with ICUD. However, there were no statistically significant differences in LOS, mortality rates, or anastomotic stricture rates between the groups. Although selection bias is an important concern when interpreting these findings, there were no differences in baseline characteristics between the groups (19).
Another similar, multi-institutional analysis found that patients undergoing ICUD experienced a lower EBL (500 vs. 400 cc; P=0.01) and a lower incidence of blood transfusions (23% vs. 5.4%; P=0.006). This study included patients who received ileal conduits and neobladders. However, on subgroup analysis including only patients who received an ileal conduit, EBL was lower in the ICUD group. There were no differences in operative time, LOS, or the need for transfusions (20).
A single-institution propensity matched analysis of ORC and RARC, with intracorporal or extracorporeal neobladders, found no significant differences in disease free survival, overall survival, or cancer specific survival at two years. However, ECUD patients experienced a higher incidence of perioperative complications (91.3% vs. 42.2%; P<0.001), most of which were due to the increased need for blood transfusions (21).
A review from the IRCC database compared patients who underwent ECUD and ICUD. The study found that patients who underwent ICUD had shorter operative times (357 vs. 400 min; P<0.001), lower EBL (300 vs.350 cc; P<0.001), and received fewer transfusions (5% vs. 13%; P<0.001). Unlike the previously mentioned studies, ICUD patients experienced more complications, especially in the first 30-days post-operatively (31% vs. 19%; P<0.001). The discrepancy in complication rates is likely explained by the fact that this analysis included patients from the early RARC experience, when the incidence of complications was significantly higher. There were no differences between the two groups in terms of LN yield and PSM. Interestingly, ICUD patients were more likely to experience peritoneal carcinomatosis (1.3% vs. 0.3%; P=0.01) and extra-pelvic LN metastasis (3% vs. 1%; P=0.01). Patients treated with ECUD experienced greater overall survival at 3- and 5-years, but the diversion approach was not significantly associated with recurrence free or disease specific survival. The observed overall survival advantage may be explained by the higher incidence of complications with ICUD, especially in the earlier stages of the ICUD experience (18).
Healthcare costs
Early hypotheses postulated that costs associated with RARC would be lower than ORC due to fewer complications and shorter LOS. However, the data have been conflicting. Using Surveillance, Epidemiology, and End Results Program (SEER) data from 2002 to 2012, Hu et al. found that RARC was associated with higher costs both during the inpatient hospital stay and at 30 days following surgery. The median inpatient cost for RARC was $24,051 compared to $21,637 for ORC, however this difference was not statistically significant. Furthermore, RARC patients had a greater likelihood of requiring home health care after hospitalization (RR 1.14) significantly raising costs at 30- and 90-day follow up (22).
By contrast, after reviewing National Medicare claims to evaluate 90-day costs following RARC, Modi et al. found that RARC was associated with significantly lower costs ($38,071 vs. $34,369) (23). The authors hypothesized that their findings differed from those of Hu et al. because the latter evaluated outcomes from the early RARC experience. It is important to note that this study was analyzing Medicare payor cost as opposed to hospital costs (23). Some studies have found higher hospital costs with RARC (24,25). One analysis estimated that for RARC to be more cost effective than ORC, it had to either prevent complications 74% of the time or prevention transfusion 70% of the time (25).
Acknowledging that there is limited data comparing cost-effectiveness of RARC versus ORC, Michels et al. constructed a model to identify current evidence gaps and the main drivers of cost-effectiveness. In this model, monetary value was assigned to various aspects of perioperative and post-operative care. For example, the cost of the OR per minute was valued at $11, and complication costs ranged from $3,160 for a Clavien 1 to $23,606 for a Clavien 5. These values were derived from a metanalysis and guidelines. Using a 90-day model with 1,000 simulations, there was a 23% probability that RARC would have lower costs and result in fewer complications. The primary drivers of cost were LOS, OR time, and equipment cost. In this study, RARC was found to me cost effective if LOS were 4 days or less, operating time were less than 175 min, and robot equipment costs were less than $317. The associated savings from a lower complication rate did not outweigh the increase in cost for RARC cases (26).
Discussion
After a review of the literature, it is our understanding that the primary benefit of RARC compared to ORC is a reduction in blood loss and blood transfusions, and the primary disadvantage is longer operative times. On multivariable analysis, perioperative blood transfusions have been associated with cancer specific and all-cause mortality, with the risk being dose-dependent (27). However, results among multiple analyses have been inconsistent (28,29). The reduced incidence of perioperative transfusions alone is likely insufficient to favor RARC over ORC. With respect to the advantages of ORC, although longer operative times correlate with an increased incidence of complications (30,31) the aforementioned metanalyses did not find any difference in the rates of complications after RARC or ORC (10,11), and so the shorter duration of ORC is of questionable clinical benefit.
In order to contextualize the debate over the potential benefits of RARC and ORC, we reviewed research comparing robot assisted radical prostatectomy (RALP) and open radical prostatectomy (ORP). Currently, RALP is the preferred approach and about 85% of all prostatectomies are performed robotically. Most studies have shown that the benefits of RALP, like RARC, include lower EBL, lower transfusion rate, and shorter hospital stay (32-34). Although other studies report equally short LOS with ORP (34), there have been no observed differences in QoL following RALP or ORC (35-37), and most studies, including an RCT, have demonstrated no difference in PSM (32,34,36,38). Costs are higher with RALP (39,40), except among surgeons who perform at least 104 procedures a year (33). Regarding complications, there is a preponderance of data supporting that RALP is associated with fewer complications than ORP. This is in stark contrast to the inconsistencies in the available outcomes data for RARC versus ORC. Overall, the advantages of RALP compared to ORP and RARC compared to ORC are similar, although the evidence that the robotic approach reduces complications is stronger for radical prostatectomy than RC.
One factor that may make complications more likely with RARC is the technical complexity. Research shows that among surgeons with previous robotic experience, there is a learning curve with a threshold of 30 procedures before PSM rates fall below 5% and a LN yield exceeds 20 nodes (41). However, in a review of the NCDB, Nielsen et al. found that the majority of radical cystectomies are performed at hospitals that average fewer than 10 cases per year and only 32% of hospitals performed 20 or more cystectomies annually. As such, it would take several years for most surgeons performing radical cystectomy to become proficient in RARC (42). Therefore, RARC is best left to experienced surgeons at high-volume centers.
Conclusions
In conclusion, we believe that both ORC and RARC are oncologically sound and feasible procedures. While there are benefits with RARC, primarily lower EBL, these benefits must be balanced against longer operative times. These findings are based on RCTs that performed only ECUD. It is unclear if performing ICUD will change these results. Finally, it is difficult to compare the costs of the two procedures and the results are muddied by the often-ill-defined distinction between costs to the hospital versus the cost to payors. However, it is likely that for both hospitals and payors, currently RARC is cost effective at high-volume centers. It is feasible that in the future, increased experience with RARC will lead to improved outcomes and justify the use of RARC over ORC. Until then, the decision to perform a robotic or open RC is best made on a patient-by-patient basis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marc C. Smaldone and Jeffrey J. Tomaszewski) for the series “Controversies in Minimally Invasive Urologic Oncology” published in Translational Andrology and Urology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.11.32). The series “Controversies in Minimally Invasive Urologic Oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int 2003;92:232-6. [Crossref] [PubMed]
- Bachman AG, Parker AA, Shaw MD, et al. Minimally invasive versus open approach for cystectomy: trends in the utilization and demographic or clinical predictors using the national cancer database. Urology 2017;103:99-105. [Crossref] [PubMed]
- Nix J, Smith A, Kurpad R, et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol 2010;57:196-201. [Crossref] [PubMed]
- Khan MS, Gan C, Ahmed K, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol 2016;69:613-21. [Crossref] [PubMed]
- Bochner BH, Sjoberg DD, Laudone VP. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med 2014;371:389-90. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur Urol 2015;67:1042-50. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Marzouk KH, et al. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. Eur Urol 2018;74:465-71. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Parekh DJ, Messer J, Fitzgerald J, et al. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol 2013;189:474-9. [Crossref] [PubMed]
- Satkunasivam R, Tallman CT, Taylor JM, et al. Robot-assisted radical cystectomy versus open radical cystectomy: a meta-analysis of oncologic, perioperative, and complication-related outcomes. Eur Urol Oncol 2019;2:443-7. [Crossref] [PubMed]
- Sathianathen NJ, Kalapara A, Frydenberg M, et al. Robotic assisted radical cystectomy vs open radical cystectomy: systematic review and meta-analysis. J Urol 2019;201:715-20. [Crossref] [PubMed]
- Tang JQ, Zhao Z, Liang Y, et al. Robotic-assisted versus open radical cystectomy in bladder cancer: a meta-analysis of four randomized controlled trails. Int J Med Robot 2018; [Crossref] [PubMed]
- Novara G, Catto JWF, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol 2015;67:376-401. [Crossref] [PubMed]
- Li K, Lin T, Fan X, et al. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev 2013;39:551-60. [Crossref] [PubMed]
- Xia L, Wang X, Xu T, et al. Robotic versus open radical cystectomy: an updated systematic review and meta-analysis. PLoS One 2015;10:e0121032 [Crossref] [PubMed]
- Son SK, Lee NR, Kang SH, et al. Safety and effectiveness of robot-assisted versus open radical cystectomy for bladder cancer: A systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2017;27:1109-20. [Crossref] [PubMed]
- Jonsson MN, Adding LC, Hosseini A, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur Urol 2011;60:1066-73. [Crossref] [PubMed]
- Hussein AA, May PR, Jing Z, et al. Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol 2018;199:1302-11. [Crossref] [PubMed]
- Tan TW, Nair R, Saad S, et al. Safe transition from extracorporeal to intracorporeal urinary diversion following robot-assisted cystectomy: a recipe for reducing operative time, blood loss and complication rates. World J Urol 2019;37:367-72. [Crossref] [PubMed]
- Lenfant L, Verhoest G, Campi R, et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional french study. World J Urol 2018;36:1711-8. [Crossref] [PubMed]
- Simone G, Tuderti G, Misuraca L, et al. Perioperative and mid-term oncologic outcomes of robotic assisted radical cystectomy with totally intracorporeal neobladder: Results of a propensity score matched comparison with open cohort from a single-centre series. Eur J Surg Oncol 2018;44:1432-8. [Crossref] [PubMed]
- Hu JC, Chughtai B, O’Malley P, et al. Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: A national comparative effectiveness study. Eur Urol 2016;70:195-202. [Crossref] [PubMed]
- Modi PK, Hollenbeck BK, Oerline M, et al. Real-world impact of minimally invasive versus open radical cystectomy on perioperative outcomes and spending. Urology 2019;125:86-91. [Crossref] [PubMed]
- Monn MF, Cary KC, Kaimakliotis HZ, et al. National trends in the utilization of robotic-assisted radical cystectomy: An analysis using the Nationwide Inpatient Sample. Urol Oncol 2014;32:785-90. [Crossref] [PubMed]
- Kukreja JB, Metcalfe MJ, Qiao W, et al. Cost-effectiveness of robot-assisted radical cystectomy using a propensity-matched cohort. Eur Urol Focus 2020;6:88-94. [Crossref] [PubMed]
- Michels CTJ, Wijburg CJ, Leijte E, et al. A cost-effectiveness modeling study of robot-assisted (RARC) versus open radical cystectomy (ORC) for bladder cancer to inform future research. Eur Urol Focus 2019;5:1058-65. [Crossref] [PubMed]
- Linder BJ, Frank I, Cheville JC, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol 2013;63:839-45. [Crossref] [PubMed]
- Morgan TM, Barocas DA, Chang SS, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol 2013;31:871-7. [Crossref] [PubMed]
- Kluth LA, Xylinas E, Rieken M, et al. Impact of peri-operative blood transfusion on the outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder. BJU Int 2014;113:393-8. [Crossref] [PubMed]
- Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: An analysis from the tennessee surgical quality collaborative. J Am Coll Surg 2015;220:550-8. [Crossref] [PubMed]
- Jackson TD, Wannares JJ, Lancaster RT, et al. Does speed matter? the impact of operative time on outcome in laparoscopic surgery. Surg Endosc 2011;25:2288-95. [Crossref] [PubMed]
- Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: A systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 2012;62:1-15. [Crossref] [PubMed]
- Leow JJ, Chang SL, Meyer CP, et al. Robot-assisted versus open radical prostatectomy: A contemporary analysis of an all-payer discharge database. Eur Urol 2016;70:837-45. [Crossref] [PubMed]
- Tosoian JJ, Loeb S. Radical retropubic prostatectomy: comparison of the open and robotic approaches for treatment of prostate cancer. Rev Urol 2012;14:20-7. [PubMed]
- Gershman B, Psutka SP, McGovern FJ, et al. Patient-reported functional outcomes following open, laparoscopic, and robotic assisted radical prostatectomy performed by high-volume surgeons at high-volume hospitals. Eur Urol Focus 2016;2:172-9. [Crossref] [PubMed]
- Herlemann A, Cowan JE, Carroll PR, et al. Community-based Outcomes of Open versus Robot-assisted Radical Prostatectomy. Eur Urol 2018;73:215-3. [Crossref] [PubMed]
- Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol 2018;19:1051-60. [Crossref] [PubMed]
- Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057-66. [Crossref] [PubMed]
- Bolenz C, Gupta A, Hotze T, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol 2010;57:453-8. [Crossref] [PubMed]
- Tomaszewski JJ, Matchett JC, Davies BJ, et al. Comparative hospital cost-analysis of open and robotic-assisted radical prostatectomy. Urology 2012;80:126-9. [Crossref] [PubMed]
- Hayn MH, Hussain A, Mansour AM, et al. The learning curve of robot-assisted radical cystectomy: Results from the international robotic cystectomy consortium. Eur Urol 2010;58:197-202. [Crossref] [PubMed]
- Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: An analysis of the National Cancer Data Base. BJU Int 2014;114:46-55. [Crossref] [PubMed]


