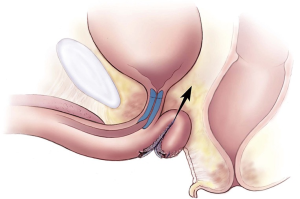
AdVance male sling
Introduction
The AdVance male sling (American Medical Systems, Minnetonka, MN, United States of America) is a synthetic transobturator sling, which is placed in a minimally invasive fashion, for the treatment of male stress urinary incontinence (SUI). It was developed by Rehder and Gozzi as a minimally invasive alternative to the Artificial Urinary Sphincter (AUS) (American Medical Systems, Minnetonka, MN, United States of America) (1). In short, sling placement involves the following steps: (I) corpus spongiosal dissection and mobilization; (II) identification and dissection of central tendon; (III) placement of needle passers through the obturator foramen; (IV) mesh placement at central tendon; (V) mesh tensioning and fixation with cystoscopy; (VI) incision closure (2).
In placing the sling, it is not only interesting but also useful to consider how the sling works: the AdVance transobturator sling contributes to male continence in several ways. When the sling is appropriately tensioned, the urethral bulb relocates a distance of 2–4 cm proximally into the pelvis. Supporting the bulb may indirectly facilitate continence in patients with post-prostatectomy incontinence (PPI) (3). The sling also provides support to the dorsal distal portion of the membranous urethra, which acts as a backstop during stress. In contrast to other slings, all this is achieved without causing any urethral obstruction (4). Rather, a mechanism of “dynamic compression” has been observed on ultrasound study (5).
In this article, we provide a step-by-step description of our technique for placement of the AdVance male sling, including details and nuances gained from surgical experience, advice for avoidance of potential surgical complications and management of complications if they occur.
Patient selection
Patient selection is very important: the AdVance sling is best suited for men with mild to moderate SUI (6). The AdVance sling has demonstrated safety and efficacy in the treatment of PPI, and can also be used in the treatment of male SUI due to other causes such as after transurethral resection of the prostate (TURP) and in men with neurogenic lower urinary tract dysfunction (NLUTD) (7-13).
It is our practice to evaluate every patient after open prostatectomy with cystourethroscopy to rule out vesicourethral anastomotic stenosis (VUAS), urethral stenoses or strictures (US), prior to placement of an AdVance sling. If urethral stricture or stenosis is present, we advise treating and stabilizing this for 3–6 months before sling placement. Typically, the patient is re-scoped 3–6 months’ after correction of VAUS or US and if the urethra remains patent, we proceed to sling insertion.
Urodynamic testing may also have a role in the evaluation of the patient with PPI (14,15). Among patients with lower urinary tract symptoms (LUTS) after radical prostatectomy (RP), 27% had detrusor overactivity (DO), 41% had detrusor underactivity (DU), and 17% had bladder outlet obstruction (BOO) (14). Patients with mixed urinary incontinence may be improved with anticholinergic, beta-3 adrenergic medication or other OAB therapy, prior to embarking on invasive surgical intervention for the stress component of their urinary incontinence. In some cases, adequate control of DO may move patients from severe range PPI to mild to moderate range PPI, and appropriate choice of continence device may be able to move from AUS to AdVance sling. DO is a negative prognostic factor for success (5,8); the presence of DU may increase the risk of urinary retention after sling insertion (16). Patients who void with Valsalva have a higher risk of loosening the sling postoperatively. Therefore, urodynamic evaluation may be beneficial for diagnostic and prognostic counselling, even if the results do not change choice of SUI procedure.
Patients with history of pelvic radiation are not good candidates for an AdVance sling. Although it is technically feasible to place a sling, we advise such patients to consider other treatment options (such as AUS). If they choose an AdVance sling, they can expect worse outcomes compared with patients who have not had any pelvic radiation (5,17-19). Radiation may limit urethral mobility and adequate proximal urethral relocation (15). It may also inhibit tissue healing and mesh ingrowth.
Men with NLUTD may be candidates for AdVance sling placement with satisfactory results (11,20,21). Evaluation and treatment of DO and low compliance should be completed prior to sling placement. Patients may have difficulty performing clean intermittent catheterization after sling placement, but changing catheter type usually overcomes this problem. Men with NLUTD and a sling in place must be committed to regular surveillance for risk of upper-tract deterioration.
Surgical technique
We perform an AdVance sling procedure with the patient under general anesthesia using laryngeal mask anesthesia—it can also be performed under spinal anesthesia. It is also important that the patient is extubated smoothly, with coughing avoided so as not to risk loosening the sling.
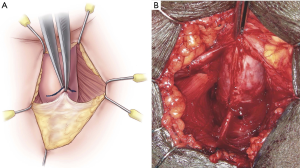
With the patient positioned in dorsal lithotomy, knees approximately shoulder-width apart and flexed no more than 90 degrees, a standard surgical prep is performed and the patient draped. A 5 cm midline perineal incision is made. The bulbospongiosus muscle is divided in its midline to expose the corpus spongiosum (CS). The CS is then mobilized distally, laterally, and proximally to the level of the central tendon. The CS at the level of the central tendon is marked either with an absorbable suture (4-0 Vicryl) or a marking pen before the central tendon is dissected off the CS (Figure 1A,B). Central tendon dissection allows for increased CS mobility during tensioning. Key points of surgical technique and further details of nuances are presented in Table 1.

Full table
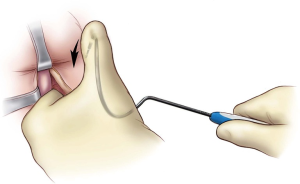
Next, bilateral stab incisions are made with a scalpel at a site 1–2 cm below the level of the adductor longus tendon just lateral to the ischiopubic ramus. This site is usually easily palpable as a ‘soft spot’, a spinal needle may be used to help identify the area of insertion. These small incisions will be sites of AdVance helical trocar entry. The trocar handle is held at a 45-degree angle, with the point of the trocar needle perpendicular to the patient’s skin, and placed straight through the small incisions (Figure 2). A finger from the surgeon’s contralateral hand is placed in the perineal incision below the ischiopubic ramus to protect the CS and guide needle placement. Two “pops” are felt; after the second “pop” the needle is turned approximately a quarter turn. The needle is palpable on the surgeon’s finger behind the ischiopubic ramus. Prior to bringing the trocar needle through the fascia, the surgeon’s ipsilateral hand is dropped, and the needle is brought out as high as possible in the apex formed by the ischiopubic ramus and CS (Figure 3). The mesh is secured to the needle and brought back through the stab incision. The trocar needle pass is repeated on the opposite side of the patient. Then, the central portion of the mesh is fixed to the CS with the proximal edge of the mesh being fixed at the level of our previous mark where the central tendon had been taken down. If this marking was lost accidentally, we can place a urethral catheter to locate the site where it makes a turn into the proximal bulbar urethra—this is a safe site to place the proximal aspect of the mesh. Two absorbable sutures (4-0 Vicryl or PDS) are placed proximally and two distally to secure the mesh to the CS (Figure 4).
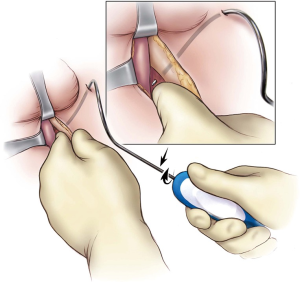
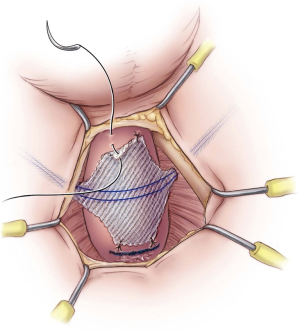
The sling is tensioned by pulling firmly on the sling arms (Figure 5). Cystourethroscopy is performed during tensioning to confirm coaptation of the external sphincter and to rule out urethral injury. If no coaptation is seen, it is usually because the sling was placed too proximal. Therefore, the four sutures are removed and the sling repositioned approximately 0.5–1 cm distally. A 14 Fr. Foley urethral catheter is inserted to remain in place overnight. The outer coverings of the mesh arms are removed, and although the sling is self-anchoring, the mesh is tunneled back subcutaneously to the midline perineal incision with the aid of a tonsil clamp to reduce the risk of slippage of the mesh. Some surgeons have proposed securing both ends of the sling together with a suture, or making a second incision beneath the first one to anchor the ends of the sling subcutaneously at a 90° angle (22). Finally, the bulbospongiosus muscle is reapproximated, and the subcutaneous tissues and perineal incision closed. The bilateral trocar incisions are closed with a simple interrupted suture.
Post-operative care
It is our practice to have patients stay in hospital overnight with a Foley urethral catheter on gravity drainage. The following morning, a void trial is conducted prior to the patient being discharged home. If the patient develops urinary retention, a 12 or 14 Fr. Foley urethral catheter is reinserted and the patient is discharged home with it attached to a leg bag. Rarely, the catheter may need to be introduced over a wire with the assistance of a cystoscope. Repeat void trial is performed at 5–7 days; if urinary retention lasts longer, subsequent void trials are performed at weekly intervals. Able patients may commence clean intermittent catheterization until adequate spontaneous bladder emptying is achieved. Risk of long term urinary retention is small. In a rare patient with retention lasting longer than 3 months, the sling may be surgically excised with the patient returning to his previous status.
Most patients experience minimal postoperative pain and may want to return to normal activity as soon as possible. However, it is imperative that the patient follows the postoperative instructions: avoid physical exertion, do not lift more than 15 lbs., do not squat or climb, for at least 6 weeks postoperatively. Patients who do not follow these instructions are at high risk of loosening their sling with return of incontinence. Patients with NLUTD and impaired mobility must be cautious not physically exert themselves during transfers.
For patients who report having done something in the first 6 weeks after sling placement which may have loosened the sling, a repeat sling placement may be performed. In these patients, re-exploration through the previous incision is performed, CS identified and dissection continued proximally until the edge of the mesh is palpable. The mesh arms are identified laterally with a right-angle clamp and transected, allowing the bulb of the CS to be pulled toward the surgeon. The broad portion of the mesh, which was attached to the CS is then excised. Another sling is then placed in correct position as described in the ‘Surgical Technique’ section of this article.
Complications
The overall complication rate for the AdVance sling was 12.3% in a systematic review (12). Severe complications, both intraoperative and postoperative, are rare (12,23). The main potential intraoperative complication is urethral injury during trocar passage. We have seen this in only two patients early in our experience.
Urinary retention is the most common early post-operative complication, occurring in 12–46% of patients; the risk of prolonged urinary retention is very low (23-25). Patients who experienced postoperative urinary retention had good continence outcomes (25). One sling was transected (0.4%) due to slippage of the sling with obstruction of the urethra (23). Other early postoperative complications include local wound infection (0.4%), febrile urinary tract infection (0.4%), persistent moderate perineal pain (0.4%) (23). There has been a case report of bleeding with hematoma, requiring blood transfusion in a patient who received early re-anticoagulation (26). There has been one case report of urethral mesh erosion in a patient with prior radiation (27). Sling explantation is also extremely rare (0.9%); in one study, an explantation was due to initial wrong placement and another due to symphysitis attributed to a Guillain-Barre Syndrome (not sling infection) (23).
Management of incontinence after AdVance sling
The failure rate after AdVance sling is 20% to 45.5% (28). In addition to evaluating the degree of further incontinence and its impact on quality of life, it is also important to differentiate between persistent incontinence and recurrent incontinence. Persistent incontinence suggests ongoing SUI (intrinsic sphincter deficiency), whereas recurrent incontinence may represent other pathology such as DO unless the patient gives clear history of having done something in the early postoperative period which may have loosened the sling. Reoperation on the outlet is only indicated in cases of ongoing SUI.
Insertion of an AUS is the ‘gold standard’ treatment after failed AdVance sling. The AdVance sling can be left in place during the salvage AUS procedure. It has been reported that up to 13% of men ultimately proceed to AUS implantation after sling surgery (29). AUS placement in this situation has a success rate of 80–90% (30). A comparison of outcomes in a group of patients who received AdVance sling then salvage AUS versus a group of patients who received virgin AUS procedures, showed that patients with salvage AUS compared favorably in terms of success and required revisions (30).
We have also performed repeat AdVance sling placement with encouraging results especially in cases of late failure (31). Success rate was 72% at 6 months and 56% at mean 17.5 months follow-up. Complication rates were low. Patients who failed late (>6 months) after their primary sling had better outcomes with salvage sling placement compared to patients who failed earlier after their primary sling (75% vs. 30%, P<0.05). This may be because patients who failed late were probably initially better candidates for sling placement than their counterparts. We believe it is also a reasonable option to offer a repeat AdVance sling placement to patients who give clear history of having initial continence success then having done something in the early postoperative period which may have loosened their sling with subsequent recurrence of incontinence.
Placement of a second AdVance sling is done in a similar manner to an initial sling placement with only several nuances in technique. We have found it slightly easier to begin the dissection further distal on the CS in a virgin plane. We mobilize the CS a little more than usual. Once the distal aspect of the sling is identified, we are able to place a right-angle clamp around the sling arms and transect them, allowing the CS to move toward the surgeon. We then excise the previous sling off the CS (Figure 6). This is usually difficult and we occasionally inadvertently enter the CS but have not injured the urethra during the dissection. Any CS openings are closed with an absorbable suture. The trocar needles are passed as previously described and the sling is placed usually slightly distal to the first sling.
Soljanik et al. reported outcomes after repeat AdVance sling whereby the first sling was left in place and did not prohibit the placement of the second sling. At mean 16.6 months follow-up, 34.5% men required no pads, 37.9% had one dry “security” pad, 3.4% one wet pad, 3.4% two pads, 10.3% pad reduction ≥50%, and 10.4% treatment failure (28).
Mild persistent incontinence after AdVance sling may also be managed with injection of urethral bulking agents, such as Macroplastique (Uroplasty, Inc., Minnetonka, MN, USA), at the area of coaptation with relatively good results.
Another option to consider would be insertion of ProACTTM balloons (Uromedica, Inc., Plymouth, MN, USA). Al-Najar et al. used the ProACTTM system in ten patients with persistent incontinence after their sling. At mean 6 months follow-up, all ten patients were pad free (32). The ProACT is not currently available in the United States of America.
AdVance XP
Since 2010, the second generation of the AdVance sling, the AdVance XP, has become available in several countries (not currently approved by the FDA in the US). It has: updated mesh waves with integrated tensioning fibers to stabilize sling configurations upon implantation; longer sling arms; the addition of chevron anchors on the sling arms, which are intended to provide enhanced acute tissue fixation; Tyvek liners to prevent the chevrons from tearing the plastic sheath; and redesigned helical needles to allow easier tunneling particularly in patients with large body habitus.
The 2-year and 36-month reports of a multi-center study of the AdVance XP sling showed good and stable effectiveness, with 66% of patients cured (using a strict definition of no pads and 0–5 g in the 24 h pad test) and 23.4% improved at 36 months (6,33). The only unique intraoperative problem reported in this study was difficulty removing the Tyvek liners in a few cases, which resulted in over-tensioning of the AdVance XP sling.
Conclusions
AdVance transobturator sling is best suited for treatment of mild to moderate SUI in male patients, and can be used in cases of PPI, post-TURP or NLUTD. Preoperative patient selection is important: it involves exclusion and prior treatment of urethral stenosis and bladder dysfunction. Patient with prior pelvic radiation have poorer outcomes. Sling placement involves the following steps: (I) mobilization of the CS and central tendon off the CS; (II) bilateral small groin incisions for passage of the helical trocar needles; (III) correct passage of the needles so they exit at the apex of the angle between the CS and inferior pubic ramus; (IV) fixation of the broad part of the sling body to the CS; (V) firm tensioning of the sling; (VI) Placement of a Foley urethral catheter; (VII) subcutaneous tunnelling of the sling arms back toward the midline; (VIII) wound closure. Surgical technique including details and nuances gained from surgical experience and advice for avoidance of complications have been described. Management of sling failures has also been discussed, including AUS, repeat sling, urethral bulking agent, and ProACT device treatment options. The AdVance XP sling is placed in similar fashion to the AdVance sling.
Acknowledgements
None.
Footnote
Conflicts of Interest: AS Chung and OA Suarez have no conflicts of interest to declare. KA McCammon is a consultant/advisor and meeting participant/lecturer for Boston Scientific; he is involved in scientific study/trials with Allergan, Astellas, Boston Scientific and Solace.
References
- Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol 2007;52:860-6. [Crossref] [PubMed]
- Rapp DE, Reynolds WS, Lucioni A, et al. Surgical technique using AdVance sling placement in the treatment of post-prostatectomy urinary incontinence. Int Braz J Urol 2007;33:231-5; discussion 236-7. [Crossref] [PubMed]
- Rehder P, Staudacher NM, Schachtner J, et al. Hypothesis That Urethral Bulb (Corpus Spongiosum) Plays an Active Role in Male Urinary Continence. Adv Urol 2016;2016:6054730. [Crossref] [PubMed]
- Davies TO, Bepple JL, McCammon KA. Urodynamic changes and initial results of the AdVance male sling. Urology 2009;74:354-7. [Crossref] [PubMed]
- Habashy D, Losco G, Tse V, et al. Mid-term outcomes of a male retro-urethral, transobturator synthetic sling for treatment of post-prostatectomy incontinence: Impact of radiotherapy and storage dysfunction. Neurourol Urodyn 2017;36:1147-50. [Crossref] [PubMed]
- Bauer RM, Grabbert MT, Klehr B, et al. 36-month data for the AdVance XP(R) male sling: results of a prospective multicentre study. BJU Int 2017;119:626-30. [Crossref] [PubMed]
- Bauer RM, Kretschmer A, Stief CG, et al. AdVance and AdVance XP slings for the treatment of post-prostatectomy incontinence. World J Urol 2015;33:145-50. [Crossref] [PubMed]
- Zuckerman JM, Edwards B, Henderson K, et al. Extended outcomes in the treatment of male stress urinary incontinence with a transobturator sling. Urology 2014;83:939-45. [Crossref] [PubMed]
- Kretschmer A, Buchner A, Leitl B, et al. Long-term Outcome of the Retrourethral Transobturator Male Sling After Transurethral Resection of the Prostate. Int Neurourol J 2016;20:335-41. [Crossref] [PubMed]
- Rehder P, Haab F, Cornu JN, et al. Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur Urol 2012;62:140-5. [Crossref] [PubMed]
- Pannek J, Wollner J. Treatment of stress urinary incontinence in men with spinal cord injury: minimally invasive=minimally effective? Spinal Cord 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Crivellaro S, Morlacco A, Bodo G, et al. Systematic review of surgical treatment of post radical prostatectomy stress urinary incontinence. Neurourol Urodyn 2016;35:875-81. [Crossref] [PubMed]
- Cornu JN, Sebe P, Ciofu C, et al. The AdVance transobturator male sling for postprostatectomy incontinence: clinical results of a prospective evaluation after a minimum follow-up of 6 months. Eur Urol 2009;56:923-7. [Crossref] [PubMed]
- Chung DE, Dillon B, Kurta J, et al. Detrusor underactivity is prevalent after radical prostatectomy: A urodynamic study including risk factors. Can Urol Assoc J 2013;7:E33-7. [Crossref] [PubMed]
- Comiter CV, Dobberfuhl AD. The artificial urinary sphincter and male sling for postprostatectomy incontinence: Which patient should get which procedure? Investig Clin Urol 2016;57:3-13. [Crossref] [PubMed]
- Comiter CV. Surgery Insight: surgical management of postprostatectomy incontinence--the artificial urinary sphincter and male sling. Nat Clin Pract Urol 2007;4:615-24. [Crossref] [PubMed]
- Zuckerman JM, Tisdale B, McCammon K. AdVance male sling in irradiated patients with stress urinary incontinence. Can J Urol 2011;18:6013-7. [PubMed]
- Torrey R, Rajeshuni N, Ruel N, et al. Radiation history affects continence outcomes after advance transobturator sling placement in patients with post-prostatectomy incontinence. Urology 2013;82:713-7. [Crossref] [PubMed]
- Bauer RM, Soljanik I, Fullhase C, et al. Results of the AdVance transobturator male sling after radical prostatectomy and adjuvant radiotherapy. Urology 2011;77:474-9. [Crossref] [PubMed]
- Groen LA, Spinoit AF, Hoebeke P, et al. The AdVance male sling as a minimally invasive treatment for intrinsic sphincter deficiency in patients with neurogenic bladder sphincter dysfunction: a pilot study. Neurourol Urodyn 2012;31:1284-7. [Crossref] [PubMed]
- Vainrib M, Reyblat P, Ginsberg D. Outcomes of Male Sling Mesh Kit Placement in Patients with Neuropathic Stress Urinary Incontinence: A Single Institution Experience. Urol Int 2015;95:406-10. [Crossref] [PubMed]
- Bauer RM, Mayer ME, Gratzke C, et al. Prospective evaluation of the functional sling suspension for male postprostatectomy stress urinary incontinence: results after 1 year. Eur Urol 2009;56:928-33. [Crossref] [PubMed]
- Bauer RM, Mayer ME, May F, et al. Complications of the AdVance transobturator male sling in the treatment of male stress urinary incontinence. Urology 2010;75:1494-8. [Crossref] [PubMed]
- Rapp DE. The male suburethral sling: remaining questions. Can J Urol 2014;21:7350. [PubMed]
- Hall M, Polland A, Weissbart S, et al. Prognostic value of postoperative urinary retention after male sling insertion. Can J Urol 2014;21:7344-9. [PubMed]
- Kruck S, Bedke J, Amend B, et al. Underestimated risk of bleeding after male transobturator sling procedure caused by early re-uptake of anticoagulation. Urol Int 2011;86:242-4. [Crossref] [PubMed]
- Harris SE, Guralnick ML, O'Connor RC. Urethral erosion of transobturator male sling. Urology 2009;73:443 e19-20.
- Soljanik I, Becker AJ, Stief CG, et al. Repeat retrourethral transobturator sling in the management of recurrent postprostatectomy stress urinary incontinence after failed first male sling. Eur Urol 2010;58:767-72. [Crossref] [PubMed]
- Kim PH, Pinheiro LC, Atoria CL, et al. Trends in the use of incontinence procedures after radical prostatectomy: a population based analysis. J Urol 2013;189:602-8. [Crossref] [PubMed]
- Lentz AC, Peterson AC, Webster GD. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J Urol 2012;187:2149-53. [Crossref] [PubMed]
- Martinez EJ, Zuckerman JM, Henderson K, et al. Evaluation of salvage male transobturator sling placement following recurrent stress urinary incontinence after failed transobturator sling. Urology 2015;85:478-82. [Crossref] [PubMed]
- Al-Najar A, Kaufmann S, Boy S, et al. Management of recurrent post-prostatectomy incontinence after previous failed retrourethral male slings. Can Urol Assoc J 2011;5:107-11. [Crossref] [PubMed]
- Bauer RM, Gozzi C, Klehr B, et al. AdVanceXP male sling: 2-year results of a multicentre study. World J Urol 2016;34:1025-30. [Crossref] [PubMed]