Why are upper tract urothelial carcinoma two different diseases?
Introduction
Urothelial carcinomas are the most common malignancies of the urinary tract and are among the most prevalent cancers worldwide. While the majority of urothelial carcinomas affect the urinary bladder, approximately ~5% of all cases are located in the upper urinary tract (1,2). Therefore, upper tract urothelial carcinoma (UTUC) has long been considered simply as a common carcinoma at a rare location. Both arising from the same tissue type, it is not surprising that upper tract urinary carcinoma share several similarities to urothelial bladder carcinoma (UBC). These similarities and its low incidence have determined the therapy of UTUC for a long time by simply adopting clinical decision making based on evidence originally gathered for UBC. In the last few years increasing body of evidence revealed the differences between UCB and UTUC including their etiology and both molecular and pathological characteristics. Therefore, it becomes increasingly evident that these two similar but distinct tumor entities require different diagnostic and therapeutic approaches. Accordingly, since 2011, the European Association of Urology submits guidelines specifically addressing the treatment of UTUC (3). The careful evaluation of their similarities and at the same time to be aware of their differences may help to further improve the management of UTUC. The aim of this review is to summarize our current knowledge on UTUC by pointing out both its similarities and differences to UBC.
Differences in epidemiology and risk factors
Urothelium is the highly specific epithelial lining of the urinary tract which includes the renal pelvis, ureters, bladder and urethra. Malignant transformation of the urothelium is frequent making urothelial carcinoma for the fourth most common tumor (1). UTUC represents a small subgroup of all urothelial carcinomas and accounts for about only 2 cases per 100,000 people each year (2).
The UTUC and UBC show an obvious difference regarding their gender disparity; while UBC occurs 3-4 times more commonly in men than in women, this ratio is 2:1 for men in UTUC (4,5). Furthermore, UBC is diagnosed at more progressed stages and has an inferior survival in women compared to men, however in contrast, no such differences were observed in UTUC (5,6). These differences may probably be explained by their anatomical location, embryological development and hormonal milieu (7).
UBC and UTUC share some common risk-factors. Similarly to UBC, smokers are at 2.5- to 7-fold elevated risk to develop UTUC compared to nonsmokers (8,9). In addition, lifetime cigarette smoking increase the risk of both tumor recurrence and disease-related death by ~25% in UBC and by ~50% in UTUC patients, showing similarities and differences at the same time (10). As UBC and UTUC are urothelial carcinomas, it is not surprising that they are mutually associated as risk factors. However, it is more likely for UBC to develop after UTUC (22–47%) (11-13) than UTUC to develop after UBC (2–6%) (14,15). Further, common risk factors for both UBC and UTUC include phenacetin and occupational risk factors such as aromatic amines, benzidine and b-naphthalene (16).
Beside the above common risk factors, UTUC also has more specific ones. The uneven geographic and familiar distribution pattern of the incidence of UTUC led to the discovery of further important risk factors. Known endemic areas of UTUC include some regions of the Balkan and Taiwan. It has been observed that in some rural villages the endemic Balkan nephropathy is strongly associated with the diagnosis of UTUC elevating its local incidence rate up to 60–100 times. The etiology of this disease however, remained largely unknown for almost 5 decades until women in Belgium were diagnosed with end-stage nephropathy following the use of aristolochic acid containing Chinese herb remedy (17,18) Finally, ~50% of them developed UTUC. Aristolochic acid, as it also produced by Aristolochia clematitis—a plant abundantly growing and frequently mixing with wheat in the affected Balkan region—represented the link between the two endemic regions. Subsequent analyses revealed that aristolochic acid caused molecular changes including the formation of aristolactam-DNA adducts in the renal cortex and specific p53 mutations at codon 139 (A:T to T:A) were present in most of these endemic patients (19). Taiwan is a further endemic region of UTUC with the world’s highest reported incidence rates (20). Also here, the use of aristolochic acid containing Chinese herbs may be accused for the increased incidence of UTUC (20). A further, however, less clear association between the “black foot disease” and UTUC may also be involved in the increased risk of UTUC in Taiwan. Black foot disease is a vasculitis caused by environmental exposure to arsenic pollution of water and its endemic areas overlap with those of UTUC (21,22).
In addition to environmental risk factors, hereditary genetic alterations may also be involved in the tumorigenesis of UTUC. Approximately 10–20% of all UTUCs have a hereditary background. It is well established that the incidence of UTUC is 8–25 fold higher in Lynch syndrome (also known as hereditary non-polyposis colorectal carcinoma; HNPCC) (23,24). The hMLH1 and hMSH2 are the most commonly damaged genes in Lynch syndrome. If both alleles of one of these genes are affected by mutation, deletion or epigenetic silencing, the mismatch-repair (MMR) function is blocked resulting in the accumulation of damaged genes ultimately leading to cancer formation with colorectal (type I) and sometimes also extra-colonic location such as ovary or upper urinary tract (type II) (24). For urothelial cancers of hereditary origin, the hMSH2 mutations are more prevalent as those of hMLH1 (25). To classify UTUC into hereditary and sporadic group the European Guidelines recommend to preform molecular analysis for UTUC patients susceptible for hereditary background based on four criteria: (I) UTUC diagnosis before the age of 60 years; (II) personal history of HNPCC-spectrum cancer; (III) at least one first-degree relative diagnosed with HNPCC under the age of 50 years; or (IV) two first-degree relatives with known HNPCC (without age restriction) (3). The molecular analysis aims to detect loss of MMR function by using immunostaining of MMR genes (hMLH1, hMSH2), DNA sequencing and microsatellite instability analysis. The loss of MMR function is associated with the resistance against chemotherapeutic agents with DNA-damaging effect (cisplatin, 5-fluorouracil, doxorubicin etc.) (26). The most frequently used radical surgical treatment of UTUC leads to the loss of kidney function that strongly limits the use of chemotherapies. Therefore, the prediction, of which patient will benefit from chemotherapy, is even more important in UTUC than in UBC.
Differences in staging
The diagnostic procedures for UTUC and UBC, using cystoscopy and cytology are similar with some important differences. Urine cytology is less sensitive for UTUC as for UBC, however, a positive cytology with a negative cystoscopy finding is strongly suggestive of UTUC (3). Nevertheless, for definitive diagnosis a positive biopsy is required. Flexible ureteroscopy is used for the visualization and sampling of UTUC. As the lamina muscularis of ureter is thick, ureteroscopic biopsies rarely contain muscle, limiting the accuracy of stage evaluation (11). This anatomical and technical limitation of ureteroscopic sampling is probably responsible for the higher frequency of upgrading and upstaging in UTUC (27-30) compared to UBC (31-33). In a retrospective study Smith et al. performed repeated biopsy after a median of 6 weeks of initial UTUC biopsy and described upstaging (from non-invasive to invasive) in 32%, while upgrading (from low grade to high grade) was observed only in 14% of patients (27). Furthermore, in contrast to UBC, tumor grade in UTUC highly correlates with stage. About 68–100% of UTUC patients with G1 tumors have a tumor stage of ≤pT1, while 62–100% of patients with G3 tumors have a ≥pT2 finding (34). Because of the inaccurate ureteroscopic staging of UTUC and the stronger correlation between grade and pathological tumor stage, it is not surprising that grade contains more relevant pathological information as tumor stage. In accordance, UTUC grade more accurately predicts survival at initial biopsy as tumor stage (35).
Various preoperative models have been constructed to predict pathological tumor stage of ureteroscopic staging. Most of these models include imaging data, biopsy staging/grading and/or cytology findings in various combinations (36-40) (Table 1). These analyses showed that the combination of selected, preoperatively available data may help to overcome the limited accuracy of ureteroscopic staging.
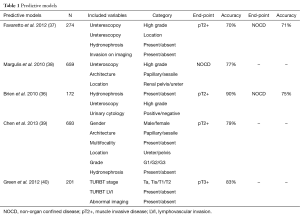
Full table
In contrast to UBC currently there is no recommended subclassification for pT3 pelvicalyceal UTUC. This led several authors to propose additional criteria for subclassification of pT3 tumors aiming to reach a more accurate risk-stratification (41-49) (Table 2). Most of the suggested criteria used various cut-offs based on the extent of tumors infiltration into the renal parenchyma and/or perirenal fat. The main limit for the first studies of this topic is that they are focusing on one single stage group with a specific location of a rare disease, necessarily limiting case numbers (14,15). Sassa (Nagoya) divided tumors to those invading the renal medulla vs. cortex, Shariat (Cornell) classified pT3 cases into microscopically vs. macroscopically renal invasive groups, while Park (Asan) categorized cases according to their invasion into renal parenchyma vs. pelvic adiposa (44,46,47). All three authors found their subclassifications prognostic for disease-specific survival (DSS) (46,47). In a subsequent study, Park et al. (43) analyzed these three proposed subclassifications in 250 pT3 pelvicalyceal UTUC cases to compare their prognostic value for recurrence-free survival (RFS) and CSS. However, all of these models proved to be prognostic, when predictive accuracy was analyzed by the Harell’s c-index only the Cornell subclassification (47) attained statistical significance (43).
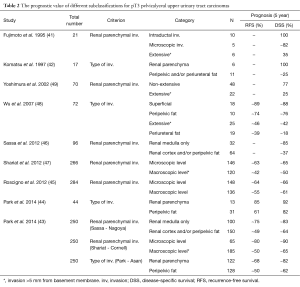
Full table
Computer tomography urography (CTU) with at least one image series in the excretory phase (10–15 min) after the administration of contrast medium is the standard imaging technique for the detection and staging of UTUC (3). Its sensitivity for UTUC detection was reported to be between 67% and 100%, while its specificity was found to be between 93% and 99% (50,51). Furthermore, its accuracy for UTUC staging was lower; 59% and 88% (52,53). Hydronephrosis and enlarged lymph nodes may also be observed on CTU images and are associated with poor patients’ prognosis (54). Magnetic resonance urography (MRU) shows a wide variability regarding its accuracy in UTUC staging, therefore it is recommended for those patients who are not eligible for CTU (3). The continuously improving imaging techniques hold the potential to overcome the limitations of biopsy in UTUC (55). Endoluminal ultrasound (56), optical coherence tomography (55), confocal laser endomicroscopy (57), multiparametric MRI (58), 11C choline PET/CT (59), narrow band imaging (60) and high definition ureteroscopy were assessed for their diagnostic and/or staging performance in UTUC in preliminary studies with limited patient numbers.
Differences in surgical treatment
Endoscopic management
Ureteroscopic resection of UTUC is limited to highly selected cases (3,34) and flexible ureteroscope is preferred over the semirigid one. Understaging and undergrading represent a significant risk when performing endoscopic surgery for UTUC (3). On the other hand, the high number of pTa cases at radical nephroureterectomy (RNU) with low risk of progression suggests that some patients would benefit from a ureter preserving treatment strategy. Improvement in risk-stratification together with the wide-spread use of flexible ureterorenoscopy made this treatment option available for patients with low-risk UTUC who are consenting to undergo strict surveillance (3,34,61,62).
Organ preservative surgery
Open nephron-sparing surgery for UTUC is mostly performed in imperative indication in patients with renal insufficiency, solitary kidney or synchronous bilateral tumors (63). Maier et al. evaluated the largest series of 55 patients undergoing nephron-sparing surgery in a multicenter protocol. They reported ~70% RFS and OS rate at a mean follow-up of over 40 months (64).
Organ preserving procedure for noninvasive low-grade tumors with proximal or mid-ureter location can be recommended, for those cases which cannot be endoscopically managed. Partial ureteral resection should be performed with wide margins to provide adequate specimens for pathological evaluation while preserving the ipsilateral kidney (3).
In patients with high-grade or invasive tumors partial ureter resection and anastomosis can be performed if renal-sparing surgery is an imperative indication when renal function preservation is the preferred goal.
Open radical surgery
For high-risk UTUC similar to UBC open radical surgery is the standard of care. However, the management of lymph nodes regarding both extent and templates are much more controversial for UTUC patients (63,65,66).
In contrast to well-established benefit of lymph node dissection (LND) during radical cystectomy, the role of LND during RNU is less obvious and therefore its performance is at the discretion of the surgeon. Only limited data are available on the impact of LND on clinical outcomes of UTUC patients. Komatsu et al. (42) analyzed 36 patients who underwent complete RNU and LND concluded that LND may offer a therapeutic advantage for selected patients with lymphatic metastasis. Furthermore, LND may help to select patients for adjuvant chemotherapy if an effective regimen is established. Miyake and coworkers (67) evaluated 72 patients who underwent RNU with or without LND for primary UTUC. They found therapeutic advantage for patients who underwent LND, especially when lymph vessel invasion was absent.
Brausi and coworkers compared the RFS and DSS in pT2-pT4 UTUC patients who underwent RNU with or without retroperitoneal LND. They found significantly better survival for patients who received LND compared to those who not. They advocated that an extended LND could be curative in patients with advanced UTUC. However, they considered neither the number of removed lymph nodes nor the template of LND (68). Similar results were found by Roscigno et al. (69) examined the role of LND on RFS and DSS in a series of 132 pT2-pT4, RNU-treated UTUC patients. The 95 patients who received LND in addition to RNU had a significant better RFS and DSS compared to those who were managed with RNU without LND. About 27% (26/95) of patients had nodal metastases in the LND group. Similar lymph node positivity rates (~28%) were found by Secin et al. in in a further LND series (70). They also showed that CT has only a limited accuracy in the prediction of positive lymph nodes (70). Further studies and a recent meta-analysis consequently found LND to provide a survival benefit only in muscle-invasive UTUC (71,72). Overall, the presence of lymph node metastasis seems to have a similar impact on survival in UTUC as in UBC patients.
Further important questions are the number of lymph nodes to be necessarily removed for accurate staging and also the sequence of LND. Roscigno found that the excision of at least 8 lymph nodes provides an independent prognostic benefit for patients and a 75% probability to find one or more positive nodes. However, this study did not consider the template of LND (73). Kondo et al., in a study on 42 node positive UTUC patients, proposed a LND template based on the location of the primary tumor (74). They proposed a more extended LND when the tumor is located to the right renal pelvis or the upper two-thirds of the ureter. In a following study, they further extended their recommendation also to interaortocaval nodes (75). Using this updated recommendation the same group in a prospective study with 77 N+M0 cases, recently demonstrated significant longer DSS and OS for patients with muscle-invasive UTUC who underwent template-based LND compared to those without LND. Template-based LND proved to be an independent prognostic factor in the subgroup of UTUC with renal pelvic location but not for tumors with ureteral involvement (75). In a recent study, Matin et al. (76) retrospectively analyzing 77 N+M0 UTUC patients confirmed that the lymphatic spread of UTUC is strongly determined by the anatomical location of the primary tumor and suggested similar LND-template as Kondo (74). Other authors also recommended to include the presacral lymph nodes in the regional lymph node template (77). Taken together, similar LND templates with only small differences have been proposed by independent studies, however, multicenter prospective analyses are still lacking to provide high grade evidence for their benefit.
Laparoscopic surgery
The number RNUs performed by laparoscopic surgery is increasing. The few available data on the long-term oncological outcomes with this surgical technique are encouraging (16). There are even more options for laparoscopic techniques than in open procedure, from hand assisted to the robotic-assisted, retroperitoneal to transperitoneal ways. The outcomes with these approaches mostly depend on the surgeon’s experience (17,18). One of the major concerns with laparoscopic RNU is the limited ability to adequately perform LND. As a consequence LND is far less frequently performed when RNU is done laparoscopically compared to open RNU (78).
Differences in medical treatment
The role of peri-operative chemotherapy is important in the treatment of UBC and UTUC. The key problem of medical treatment of UTUC is that it mainly derived from experiences initially made on bladder cancer. For both locations the discussion should be divided for superficial/non-muscle invasive and advanced/muscle invasive groups. In non-muscle invasive bladder cancer intravesical chemotherapy and immunotherapy are essential components of standard care to avoid recurrence and progression (79). More than one-third of endoscopically treated UTUCs will recur. Other authors reported up to 70% recurrence rate in the upper tract after kidney sparing surgery (80). To reduce recurrence adjuvant immunotherapy or chemotherapy can be used.
The methods to achieve appropriate concentrations of immuno/chemotherapy agents during instillation in UTUC are difficult. Infusion through a percutaneous nephrostomy tube, retrograde ureteral catheter or retrograde reflux from the bladder with an indwelling double-J stent is all acceptable methods for the instillation. The aim of the treatment is a continued exposure of urothelium to the local agent while maintaining a low-pressure system that is free of infection (63). For the anterograde instillation a nephrostomy should be inserted for the whole period of adjuvant treatment, which impairs the patients’ quality of life. The intrarenal pressure should keep below 25 cmH2O during antegrade filling. The main criticism of percutaneous instillation is the risk of tumor recurrence through seeding of cancer cells at the puncture site. However, it seems only to be a theoretical risk since seeding after adjuvant percutaneous therapy has not been reported yet in the literature (81). Retrograde instillation of topical agent using an indwelling double-J stent is advised to start with a cystogram performed in Trendelenburg position in order to determine the volume necessary to reach appropriate retrograde reflux from the bladder (82). According to Yossepowitch et al., only 59% of patients had reflux after cystography in the presence of a double-J stent (83). The major concern with ureteric stents is the increased risk of mucosa injury and the possibility of ureteric obstruction that will lead to a subsequent pyelovenous influx during instillation. With a perfect instillation technique the topical agent should remain at the tumor site for a sufficient period to cause a satisfactory antitumor effect, with a relative short duration of exposure.
Some types of intravesical therapies used to treat UBC can also be used for UTUC. Several chemotherapeutic and immunomodulatory agents have been tested in the upper tract: BCG, mitomycin C, epirubicin, thiotepa, BCG/interferon (84). The most common agents instilled are BCG or mitomycin C (85). Eastham et al. examined the use of mitomycin C after endoscopic resection of superficial UTUC. Systemic side effects were not seen with mitomycin C perfusion. Five out of seven patients had no evidence of the disease (86). According to Martínez-Piñeiro et al., the recurrence rate of UTUC treated by endourological approach decrease from 24% to 12.5% with adjuvant BCG instillation and to 14% with adjuvant mitomycin C instillation (87). In a retrospective analysis, Thalmann et al. found no tumor seeding along the nephrostomy tract and BCG therapy did not alter renal function. The authors concluded that BCG therapy of papillary UTUC does not prevent recurrence or progression but might prevent high risk patients from dialysis. However, BCG did provide cure in approximately 50% in CIS of the upper urinary tract (88). Katz et al. reported their results with BCG and interferon-α2B therapy using a retrograde instillation for UTUC. Eight patients (80%) demonstrated a complete response to therapy, and 2 had a partial response without any side effects or complications (89). Giannarini et al. showed 40% recurrence and 5% progression rate when treating CIS in the upper urinary tract and no encouraging results for Ta/T1 lesions with the same method (90). Similarly, Rastinehad et al. showed no significant benefit with adjuvant BCG perfusion in Ta/T1 tumors (81). Jabbour et al. found lower recurrence rate in grade 1 tumors treated with BCG (14%) compared to the control, non BCG group (50%) (91). According to Kojima et al. topical instillation of BCG can be used with curative intent in CIS, as the authors concluded that BCG therapy was as effective as RNU in the long-term outcomes (92). Topical instillations of the upper urinary tract appear to be safe as the major complications like BCG dissemination and secondary urosepsis are relatively rare. Renal function was not impaired after instillations of BCG or mitomycin C, preserving patients’ quality of life (93).
In muscle-invasive UBC intravenous chemotherapy is important in the standard of care. Data from a randomized phase III study demonstrated a survival advantage for neoadjuvant cisplatin-based combination chemotherapy in UBC (94). According to consensus opinion patients with pT3–4 disease or lymph node involvement likely benefit from adjuvant chemotherapy (63). Because of the low incidence of advanced UTUC, neoadjuvant and adjuvant chemotherapy settings are based on experience from UBC. There are two major components in the decision making for these patients. First, the loss of renal function that occurs with RNU can alter the administration of appropriate postoperative cisplatin-based chemotherapy. Before nephrectomy only 49% of patients have a GFR that allows cisplatin based chemotherapy and this decreases to 19% after nephrectomy. Second, before neoadjuvant chemotherapy patients are just clinically staged and there is a risk for upstaging or downstaging on final pathologic evaluation.
The goal of neoadjuvant chemotherapy is to cure subclinical metastases and decrease the size of primary tumor. Patients tolerate much better chemotherapy before surgery and higher doses can be delivered for them, but neoadjuvant chemotherapy may have a negative effect on the non-responder patients due to the delayed surgery. Igawa et al. showed an 53% overall response rate of using cisplatin-based neoadjuvant chemotherapy (MVAC, MEC, MVEC) in locally advanced UTUC (95). Matin et al. reported significantly higher pathologic downstaging and 14% complete response with neoadjuvant chemotherapy in high grade UTUC patients (96). Porten et al. showed significant improvement in OS and DSS with neoadjuvant chemotherapy compared to RNU alone (97).
On the other hand, adjuvant chemotherapy appears to have a limited role in advanced UTUC treatment. Adjuvant chemotherapy can achieve a recurrence-free rate ≤50% (98). Lee et al. reported no significant differences in RFS and DSS between adjuvant chemotherapy and control groups for pT3N0M0 UTUC patients (99). Soga et al. published significantly lower recurrence rate in the adjuvant MVAC chemotherapy UTUC patients, compared to the control group (100). Kwak et al. demonstrated the therapeutic benefit of adjuvant chemotherapy in invasive but non-metastatic UTUC patients, where the RFS rate was higher in the chemotherapy group (101). Hellenthal et al. showed a retrospective data analysis of T3–4 and/or node positive, non-metastatic UTUC patients. There was no significant difference in OS or DSS between adjuvant chemotherapy and control group (102). Thus specific clinical characteristics of each individual patient (renal function, comorbidities, tumor location, grade, stage and molecular markers) should take into account at the decision making. Recent studies suggested that adjuvant chemotherapy may provide benefit only for high risk patients with pT3–4 UTUC and lymph node involvement (103). Further prospective trials are needed to assess the role of perioperative chemotherapy in advanced UTUC and also studies with concurrent chemoradiotherapy are eagerly awaited.
Conclusions
UTUC and UBC are two different tumor entities with many important similarities. These similarities on the one hand providing advantages, as some diagnostic and treatment methods can be adapted more easily from UBC, but on the other hand, disregarding the characteristic differences between UTUC and UBC may result in treatment failure.
Current research gained more insight into the characteristics of UTUC providing more detailed information for optimal treatment-decision. Multiple predicting models may help to overcome the inaccuracy of ureteroscopic staging which represents one of the major problems of UTUC management. A more accurate preoperative risk stratification may help to select patients for kidney-sparing surgery. Further collaborative clinical studies are urgently needed to evaluate the benefit of LND and chemotherapy in UTUC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000;164:1523-5. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol 2015;68:868-79. [Crossref] [PubMed]
- Fajkovic H, Halpern JA, Cha EK, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 2011;29:457-63. [Crossref] [PubMed]
- McLaughlin JK, Silverman DT, Hsing AW, et al. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res 1992;52:254-7. [PubMed]
- Scosyrev E, Noyes K, Feng C, et al. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68-74. [Crossref] [PubMed]
- Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013;189:1214-21. [Crossref] [PubMed]
- Colin P, Koenig P, Ouzzane A, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int 2009;104:1436-40. [Crossref] [PubMed]
- Shariat SF, Favaretto RL, Gupta A, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2011;29:481-6. [Crossref] [PubMed]
- van Osch FH, Jochems SH, van Schooten FJ, et al. Significant role of lifetime cigarette smoking in worsening bladder cancer and upper tract urothelial carcinoma prognosis: a meta-analysis. J Urol 2016;195:872-9. [Crossref] [PubMed]
- Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of metachronous bladder transitional cell carcinoma (TCC) after nephroureterectomy for TCC of the upper urinary tract. BJU Int 2008;101:1368-74. [Crossref] [PubMed]
- Xylinas E, Rink M, Margulis V, et al. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol 2012;61:1069-70. [Crossref] [PubMed]
- Zigeuner RE, Hutterer G, Chromecki T, et al. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int 2006;98:1181-6. [Crossref] [PubMed]
- Li WM, Shen JT, Li CC, et al. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol 2010;57:963-9. [Crossref] [PubMed]
- Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 2009;16:187-91. [Crossref] [PubMed]
- Wilson RT, Donahue M, Gridley G, et al. Shared occupational risks for transitional cell cancer of the bladder and renal pelvis among men and women in Sweden. Am J Ind Med 2008;51:83-99. [Crossref] [PubMed]
- Cosyns JP, Jadoul M, Squifflet JP, et al. Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int 1994;45:1680-8. [Crossref] [PubMed]
- Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 2000;342:1686-92. [Crossref] [PubMed]
- Moriya M, Slade N, Brdar B, et al. TP53 Mutational signature for aristolochic acid: an environmental carcinogen. Int J Cancer 2011;129:1532-6. [Crossref] [PubMed]
- Lai MN, Wang SM, Chen PC, et al. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst 2010;102:179-86. [Crossref] [PubMed]
- Tan LB, Chen KT, Guo HR. Clinical and epidemiological features of patients with genitourinary tract tumour in a blackfoot disease endemic area of Taiwan. BJU Int 2008;102:48-54. [Crossref] [PubMed]
- Yang MH, Chen KK, Yen CC, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002;59:681-7. [Crossref] [PubMed]
- Sijmons RH, Kiemeney LA, Witjes JA, et al. Urinary tract cancer and hereditary nonpolyposis colorectal cancer: risks and screening options. J Urol 1998;160:466-70. [Crossref] [PubMed]
- Watson P, Lynch HT. The tumor spectrum in HNPCC. Anticancer Res 1994;14:1635-9. [PubMed]
- Engel C, Loeffler M, Steinke V, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol 2012;30:4409-15. [Crossref] [PubMed]
- Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res 1998;4:1-6. [PubMed]
- Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology 2011;78:82-6. [Crossref] [PubMed]
- Weizer AZ, Faerber GJ, Wolf JS Jr. Progression of disease despite good endoscopic local control of upper tract urothelial carcinoma. Urology 2007;70:469-72. [Crossref] [PubMed]
- Thompson RH, Krambeck AE, Lohse CM, et al. Endoscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. Urology 2008;71:713-7. [Crossref] [PubMed]
- Iborra I, Solsona E, Casanova J, et al. Conservative elective treatment of upper urinary tract tumors: a multivariate analysis of prognostic factors for recurrence and progression. J Urol. 2003;169:82-5. [Crossref] [PubMed]
- Herr HW, Donat SM, Reuter VE. Management of low grade papillary bladder tumors. J Urol 2007;178:1201-5; discussion 1205. [Crossref] [PubMed]
- Holmäng S, Andius P, Hedelin H, et al. Stage progression in Ta papillary urothelial tumors: relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol 2001;165:1124-8; discussion 1128-30. [Crossref] [PubMed]
- Pruthi RS, Baldwin N, Bhalani V, et al. Conservative management of low risk superficial bladder tumors. J Urol 2008;179:87-90; discussion 90. [Crossref] [PubMed]
- Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012;110:614-28. [Crossref] [PubMed]
- Grasso M, Fishman AI, Cohen J, et al. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int 2012;110:1618-26. [Crossref] [PubMed]
- Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184:69-73. [Crossref] [PubMed]
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int 2012;109:77-82. [Crossref] [PubMed]
- Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 2010;184:453-8. [Crossref] [PubMed]
- Chen XP, Xiong GY, Li XS, et al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China. BJU Int 2013;112:917-24. [PubMed]
- Green DA, Rink M, Hansen J, et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int 2013;111:404-11. [Crossref] [PubMed]
- Fujimoto H, Tobisu K, Sakamoto M, et al. Intraductal tumor involvement and renal parenchymal invasion of transitional cell carcinoma in the renal pelvis. J Urol 1995;153:57-60. [Crossref] [PubMed]
- Komatsu H, Tanabe N, Kubodera S, et al. The role of lymphadenectomy in the treatment of transitional cell carcinoma of the upper urinary tract. J Urol 1997;157:1622-4. [Crossref] [PubMed]
- Park J, Habuchi T, Arai Y, et al. Reassessment of prognostic heterogeneity of pT3 renal pelvic urothelial carcinoma: analysis in terms of proposed pT3 subclassification systems. J Urol 2014;192:1064-71. [Crossref] [PubMed]
- Park J, Park S, Song C, et al. Peripelvic/periureteral fat invasion is independently associated with worse prognosis in pT3 upper tract urothelial carcinoma. World J Urol 2014;32:157-63. [Crossref] [PubMed]
- Roscigno M, Cha EK, Rink M, et al. International validation of the prognostic value of subclassification for AJCC stage pT3 upper tract urothelial carcinoma of the renal pelvis. BJU Int 2012;110:674-81. [Crossref] [PubMed]
- Sassa N, Tsuzuki T, Fukatsu A, et al. Is pT3 urothelial carcinoma of the renal pelvis a homogeneous disease entity? Proposal for a new subcategory of the pT3 classification. Histopathology 2012;61:620-8. [PubMed]
- Shariat SF, Zigeuner R, Rink M, et al. Subclassification of pT3 urothelial carcinoma of the renal pelvicalyceal system is associated with recurrence-free and cancer-specific survival: proposal for a revision of the current TNM classification. Eur Urol 2012;62:224-31. [Crossref] [PubMed]
- Wu CF, Pang ST, Chen CS, et al. The impact factors on prognosis of patients with pT3 upper urinary tract transitional cell carcinoma. J Urol 2007;178:446-50, dicussion 450.
- Yoshimura K, Arai Y, Fujimoto H, et al. Prognostic impact of extensive parenchymal invasion pattern in pT3 renal pelvic transitional cell carcinoma. Cancer 2002;94:3150-6. [Crossref] [PubMed]
- Wang LJ, Wong YC, Huang CC, et al. Multidetector computerized tomography urography is more accurate than excretory urography for diagnosing transitional cell carcinoma of the upper urinary tract in adults with hematuria. J Urol 2010;183:48-55. [Crossref] [PubMed]
- Jinzaki M, Matsumoto K, Kikuchi E, et al. Comparison of CT urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. AJR Am J Roentgenol 2011;196:1102-9. [Crossref] [PubMed]
- Scolieri MJ, Paik ML, Brown SL, et al. Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma. Urology 2000;56:930-4. [Crossref] [PubMed]
- Fritz GA, Schoellnast H, Deutschmann HA, et al. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur Radiol 2006;16:1244-52. [Crossref] [PubMed]
- Chung PH, Krabbe LM, Darwish OM, et al. Degree of hydronephrosis predicts adverse pathological features and worse oncologic outcomes in patients with high-grade urothelial carcinoma of the upper urinary tract. Urol Oncol 2014;32:981-8. [Crossref] [PubMed]
- Bus MT, de Bruin DM, Faber DJ, et al. Optical diagnostics for upper urinary tract urothelial cancer: technology, thresholds, and clinical applications. J Endourol 2015;29:113-23. [Crossref] [PubMed]
- Matin SF, Kamat AM, Grossman HB. High-frequency endoluminal ultrasonography as an aid to the staging of upper tract urothelial carcinoma: imaging findings and pathologic correlation. J Ultrasound Med. 2010;29:1277-84. [PubMed]
- Bui D, Mach KE, Zlatev DV, et al. A pilot study of in vivo confocal laser endomicroscopy of upper tract urothelial carcinoma. J Endourol 2015;29:1418-23. [Crossref] [PubMed]
- Yoshida S, Kobayashi S, Koga F, et al. Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol 2013;23:2206-14. [Crossref] [PubMed]
- Sassa N, Kato K, Abe S, et al. Evaluation of 11C-choline PET/CT for primary diagnosis and staging of urothelial carcinoma of the upper urinary tract: a pilot study. Eur J Nucl Med Mol Imaging 2014;41:2232-41. [Crossref] [PubMed]
- Traxer O, Geavlete B, de Medina SG, et al. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol 2011;25:19-23. [Crossref] [PubMed]
- Rouprêt M, Traxer O, Tligui M, et al. Upper urinary tract transitional cell carcinoma: recurrence rate after percutaneous endoscopic resection. Eur Urol 2007;51:709-13; discussion 714. [Crossref] [PubMed]
- Rink M, Xylinas E, Margulis V, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol 2013;63:1082-90. [Crossref] [PubMed]
- Cai G, Liu X, Wu B. Treatment of upper urinary tract urothelial carcinoma. Surg Oncol 2011;20:43-55. [Crossref] [PubMed]
- Maier U, Mertl G, Pummer K, et al. Organ-preserving surgery in patients with urothelial tumors of the upper urinary tract. Eur Urol 1990;18:197-200. [PubMed]
- Arancibia MF, Bolenz C, Michel MS, et al. The modern management of upper tract urothelial cancer: surgical treatment. BJU Int 2007;99:978-81. [Crossref] [PubMed]
- Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol 2007;4:432-43. [Crossref] [PubMed]
- Miyake H, Hara I, Gohji K, et al. The significance of lymphadenectomy in transitional cell carcinoma of the upper urinary tract. Br J Urol 1998;82:494-8. [Crossref] [PubMed]
- Brausi MA, Gavioli M, De Luca G, et al. Retroperitoneal lymph node dissection (RPLD) in conjunction with nephroureterectomy in the treatment of infiltrative transitional cell carcinoma (TCC) of the upper urinary tract: impact on survival. Eur Urol 2007;52:1414-8. [Crossref] [PubMed]
- Roscigno M, Cozzarini C, Bertini R, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol 2008;53:794-802. [Crossref] [PubMed]
- Secin FP, Koppie TM, Salamanca JI, et al. Evaluation of regional lymph node dissection in patients with upper urinary tract urothelial cancer. Int J Urol 2007;14:26-32. [Crossref] [PubMed]
- Burger M, Shariat SF, Fritsche HM, et al. No overt influence of lymphadenectomy on cancer-specific survival in organ-confined versus locally advanced upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy: a retrospective international, multi-institutional study. World J Urol 2011;29:465-72. [Crossref] [PubMed]
- Roscigno M, Shariat SF, Margulis V, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol 2009;181:2482-9. [Crossref] [PubMed]
- Roscigno M, Shariat SF, Freschi M, et al. Assessment of the minimum number of lymph nodes needed to detect lymph node invasion at radical nephroureterectomy in patients with upper tract urothelial cancer. Urology 2009;74:1070-4. [Crossref] [PubMed]
- Kondo T, Nakazawa H, Ito F, et al. Impact of the extent of regional lymphadenectomy on the survival of patients with urothelial carcinoma of the upper urinary tract. J Urol 2007;178:1212-7; discussion 1217. [Crossref] [PubMed]
- Kondo T, Hashimoto Y, Kobayashi H, et al. Template-based lymphadenectomy in urothelial carcinoma of the upper urinary tract: impact on patient survival. Int J Urol 2010;17:848-54. [Crossref] [PubMed]
- Matin SF, Sfakianos JP, Espiritu PN, et al. Patterns of lymphatic metastases in upper tract urothelial carcinoma and proposed dissection templates. J Urol 2015;194:1567-74. [Crossref] [PubMed]
- Abe T, Takada N, Matsumoto R, et al. Outcome of regional lymphadenectomy in accordance with primary tumor location on laparoscopic nephroureterectomy for urothelial carcinoma of the upper urinary tract: a prospective study. J Endourol 2015;29:304-9. [Crossref] [PubMed]
- Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol 2009;56:1-9. [Crossref] [PubMed]
- Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639-53. [Crossref] [PubMed]
- Yakoubi R, Colin P, Seisen T, et al. Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma: a meta-analysis and a systematic review of current evidence from comparative studies. Eur J Surg Oncol 2014;40:1629-34. [Crossref] [PubMed]
- Rastinehad AR, Ost MC, Vanderbrink BA, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guérin therapy? Urology 2009;73:27-31. [Crossref] [PubMed]
- Irie A, Iwamura M, Kadowaki K, et al. Intravesical instillation of bacille Calmette-Guérin for carcinoma in situ of the urothelium involving the upper urinary tract using vesicoureteral reflux created by a double-pigtail catheter. Urology 2002;59:53-7. [Crossref] [PubMed]
- Yossepowitch O, Lifshitz DA, Dekel Y, et al. Assessment of vesicoureteral reflux in patients with self-retaining ureteral stents: implications for upper urinary tract instillation. J Urol 2005;173:890-3. [Crossref] [PubMed]
- Audenet F, Traxer O, Bensalah K, et al. Upper urinary tract instillations in the treatment of urothelial carcinomas: a review of technical constraints and outcomes. World J Urol 2013;31:45-52. [Crossref] [PubMed]
- Koukourakis G, Zacharias G, Koukourakis M, et al. Comprehensive management of upper tract urothelial carcinoma. Adv Urol 2009.656521. [PubMed]
- Eastham JA, Huffman JL. Technique of mitomycin C instillation in the treatment of upper urinary tract urothelial tumors. J Urol 1993;150:324-5. [PubMed]
- Martínez-Piñeiro JA, García Matres MJ, Martínez-Piñeiro L. Endourological treatment of upper tract urothelial carcinomas: analysis of a series of 59 tumors. J Urol 1996;156:377-85. [Crossref] [PubMed]
- Thalmann GN, Markwalder R, Walter B, et al. Long-term experience with bacillus Calmette-Guerin therapy of upper urinary tract transitional cell carcinoma in patients not eligible for surgery. J Urol 2002;168:1381-5. [Crossref] [PubMed]
- Katz MH, Lee MW, Gupta M. Setting a new standard for topical therapy of upper-tract transitional-cell carcinoma: BCG and interferon-alpha2B. J Endourol 2007;21:374-7; discussion 377. [Crossref] [PubMed]
- Giannarini G, Kessler TM, Birkhäuser FD, et al. Antegrade perfusion with bacillus Calmette-Guérin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: who may benefit? Eur Urol 2011;60:955-60. [Crossref] [PubMed]
- Jabbour ME, Smith AD. Primary percutaneous approach to upper urinary tract transitional cell carcinoma. Urol Clin North Am 2000;27:739-50. [Crossref] [PubMed]
- Kojima Y, Tozawa K, Kawai N, et al. Long-term outcome of upper urinary tract carcinoma in situ: effectiveness of nephroureterectomy versus bacillus Calmette-Guérin therapy. Int J Urol 2006;13:340-4. [Crossref] [PubMed]
- Jarrett TW, Sweetser PM, Weiss GH, et al. Percutaneous management of transitional cell carcinoma of the renal collecting system: 9-year experience. J Urol 1995;154:1629-35. [Crossref] [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014;65:778-92. [Crossref] [PubMed]
- Igawa M, Urakami S, Shiina H, et al. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol Int 1995;55:74-7. [Crossref] [PubMed]
- Matin SF, Margulis V, Kamat A, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 2010;116:3127-34. [Crossref] [PubMed]
- Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014;120:1794-9. [Crossref] [PubMed]
- Vassilakopoulou M, de la Motte Rouge T, Colin P, et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer 2011;117:5500-8. [Crossref] [PubMed]
- Lee SE, Byun SS, Park YH, et al. Adjuvant chemotherapy in the management of pT3N0M0 transitional cell carcinoma of the upper urinary tract. Urol Int 2006;77:22-6. [Crossref] [PubMed]
- Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol 2008;15:800-3. [Crossref] [PubMed]
- Kwak C, Lee SE, Jeong IG, et al. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology 2006;68:53-7. [Crossref] [PubMed]
- Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol 2009;182:900-6. [Crossref] [PubMed]
- Mathieu R, Bensalah K, Lucca I, et al. Upper urinary tract disease: what we know today and unmet needs. Transl Androl Urol 2015;4:261-72. [PubMed]


