Can lifestyle modification affect men’s erectile function?
Introduction
Awareness of erectile dysfunction (ED) has increased since FDA approval of sildenafil in 1998 and the subsequent escalation of direct-to-consumer advertising of phosphodiesterase inhibitors (PDE5i) (1,2). In 2005, sildenafil and tadalafil were each listed in the top 20 pharmaceutical products with regard to spending on direct-to-consumer advertising. Together, direct-to-consumer advertising for these two medications in 2005 cost $190 million (1,3). Given ubiquitous advertising and patient recognition, ED has become a condition commonly managed not only by urologists, but primary care physicians as well.
ED is a common concern for men and their partners, can cause significant depression and anxiety, and can greatly impact quality of life (QoL) (4,5). ED can also lead to a lower level of physical and emotional intimacy resulting in a lower level of satisfaction within a relationship. Data from various studies have estimated that as many as half of men aged 40–70 years old have some form of ED (2,6-9). It is further estimated that 10% of men aged 30–39 have ED with prevalence increasing to 59% of men aged 70–79 (8). This extrapolates to over twenty million men in the United States alone with ED. Notably, prevalence of severe ED increases sharply with age, with >35% of men over age 70 reporting difficulty in obtaining or maintaining erections (7). Globally, ED is predicted to affect more than 300 million men worldwide by 2025 (2). It is these staggering estimations that have made ED a broad public health concern within a globally ageing population.
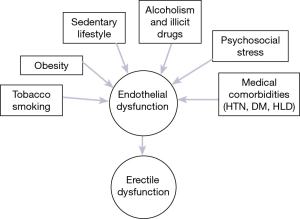
There are now well-established pathophysiologic and epidemiologic links between ED and risk factors for cardiovascular disease (CVD) such as hypertension, hyperlipidemia and diabetes (6,10). This relationship was demonstrated in the Massachusetts Male Aging Study (MMAS) and subsequently corroborated in further large-scale epidemiologic studies (6-8,10,11). Pathophysiologically, endothelial dysfunction is considered to be the underlying mechanism common to CVD and ED (Figure 1) (12,13). It follows that ED has been associated with an increased risk of premature mortality (14). The recognition of this association has prompted recommendations by the Princeton Consensus Conference for the thorough evaluation and management of cardiovascular risk in all patients presenting with ED and no known CVD (15).
Importantly, sequelae of ED are known to extend beyond physical and sexual health. ED is also known to cause detriment to QoL, psychosocial and emotional well-being for both the patient and his partner (5,16). In pretreatment screening of patients with ED and depressive symptoms on the Beck Depression Inventory-II, severity of ED was found to be predictive of depression (17). Controlled clinical trials have demonstrated improvement in psychological outcomes including confidence, sexual satisfaction and symptoms of depression following treatment with pharmacologic agents (18-21). Additionally, change in penile rigidity after treatment for ED has been associated with improvement in sexual function and QoL in female partners (22). Thus, prevention and treatment of ED represents an important means to improve patient and partner wellness and overall men’s health.
Previous publications have recognized modifiable lifestyle factors such as obesity, physical activity, smoking, diet and others as major contributors to the onset and evolution of both CVD and ED (8,9,23). Guidelines developed during the 2009 International Consultation on Sexual Dysfunction included “lifestyle modification” as a foundational step in the treatment algorithm of ED (23,24). However, patient knowledge about modifiable risk factors for ED, in particular smoking, control of CVD risk factors and sedentary lifestyle, is poor, and specific recommendations regarding implementation of lifestyle modification have not previously been outlined (25). Additionally, questions remain as to the quantitative effects lifestyle modification and supplemental therapies can have on the natural history of ED.
The aim of this review is to delineate lifestyle choices which may impose an increased risk of developing ED, present relevant studies addressing behavioral factors correlated with ED, as well as highlight proposed mechanisms for intervention aimed at improving erectile function in men with ED.
Smoking
Smoking has been shown in several studies to be positively associated with an increased risk of ED. Longitudinal epidemiologic studies have reported a relative risk of developing ED 1.5–2 times more in smokers in comparison to non-smokers (7,8,26,27). In the Boston Area Community Health survey, a cross-sectional study of 2,301 men, a dose-response relationship was demonstrated between smoking and ED (28). Significance was achieved at 20-pack years cumulative exposure after adjusting for risk factors of age, CVD, and diabetes. Though not found to be significant, passive smoking exposure trended toward a significant risk of ED. While this study design is subject to recall bias, it may provide important information when quantifying risk of ED due to smoking exposure.
Positive dose-response association between quantity and duration of smoking with risk of ED was confirmed in a meta-analysis of observational epidemiologic studies (29). The investigators found an incremental increased risk of ED per 10 cigarettes smoked per day and 10 years of smoking, by 14% and 15%, respectively. An individualized inverse dose-response relationship was seen in male smokers undergoing polysomnographic assessment of nocturnal penile tumescence (NPT), where the highest consumers of cigarettes (>40 cigarettes per day) had the fewest minutes of nocturnal tumescence and detumesced fastest (30). At a molecular and cellular level in the animal model, cigarette smoking (CS) is linked to significantly higher markers of oxidative stress and cavernosal tissue apoptosis (31). CS exposed rats were noted to have significantly lower expression of cavernosal neuronal nitric oxide synthase (nNOS) and decreased endothelial and smooth muscle content, supporting the role of endothelial dysfunction in pathophysiology of ED (12).
The effect of smoking cessation on erectile function has also been examined. Pourmand et al. prospectively studied a sample of men with ED and smoking as their only risk factor; excluded were men with other risk factors for ED such as diabetes, hypertension, dyslipidemia, peripheral vascular disease, psychiatric disorders, and renal failure. At baseline, severity of ED was found to be significantly correlated to duration of exposure in pack-years (32). At follow-up 1 year after smoking cessation, patients who successfully stopped smoking (ex-smokers) had a 25% improvement in erectile function, while men who continued (current smokers) did not improve. Additionally, a larger proportion of current smokers (7%) than ex-smokers (2.5%) had worsening of their baseline ED. This study suggests a large degree of stabilization or improvement in ED after smoking cessation.
These results were corroborated in a randomized controlled study of Chinese men enrolled in a nicotine replacement therapy (NRT) program with or without counseling. Six months after enrollment, patients who successfully quit smoking were more likely to have improvement in erectile function compared to persistent smokers (53.8% vs. 28.1%, P<0.001; RR =2.10; 95% CI, 1.64–2.70) (33). Smoking cessation was also shown to rapidly improve penile hemodynamic parameters in a group of 20 men with ED, with a significant decrease in end-diastolic velocity (EDV) and a trend toward improvement in peak systolic velocity (PSV) 24 to 36 h after smoking cessation (34).
Lastly, Harte et al. examined changes in penile tumescence in a cohort of young men (mean age ≤40 years) regardless of erectile function before and after smoking cessation (35). Using an intention-to-treat analysis, the authors demonstrated significantly reduced time to maximal erection and a greater percentage change of penile tumescence at follow-up between successful quitters compared with unsuccessful quitters. This study highlights the physiologic benefit of smoking cessation in young men, irrespective of erectile function, which, clinically, can serve as a relevant motivator for early cessation.
The importance of smoking cessation counseling during initial evaluation and treatment of ED cannot be understated. Awareness of the smoking cessation strategies such as nicotine replacement, pharmacologic adjuncts (i.e., bupropion, varenicline), cessation programs, support, and maintenance groups is a critical part of the Urologists armamentarium in ED counseling (36,37).
Obesity
Obesity is believed to directly impact erectile function by producing a chronic inflammatory state, oxidative stress and ultimately, endothelial dysfunction (12). From an epidemiologic standpoint, the Health Professionals Follow-up Study (HPFS) showed a linear relationship between increasing body mass index (BMI) and ED risk. Obese men (BMI ≥30 kg/m2) were twice as likely to have ED as men in normal weight range (BMI <25 kg/m2) (8).
The effect of weight loss on erectile function was studied in randomized-controlled trial of 110 obese men with ED (38). Fifty-five men in the intervention group received intensive, individualized instruction on nutrition and physical activity, while 55 men received general information on diet and exercise. Baseline mean IIEF scores between the groups were similar at 13.9 and 13.5 (P=0.55), respectively. After 2 years of follow-up, 31% of men in the intervention group regained normal erectile function, having improved IIEF-5 score to ≥22. BMI and physical activity level were both shown to be independent predictors of IIEF score. Of note, subjects in the intervention group were also noted to have significant decreases in serum IL-6 and CRP, both markers of inflammation and surrogate markers for endothelial dysfunction (39). It is important to note, intensive dietary and exercise instruction with close personal follow up is not always feasible in the clinical setting.
Esposito et al. randomized 65 men with ED (IIEF-5 ≤21) and metabolic syndrome [as diagnosed by recommendations in the adult treatment panel III (40)] to two groups: an individualized dietary intervention group with intensive instruction on the Mediterranean diet (rich in whole grains, fruits, vegetables, legumes, walnuts and olive oil); and a control group who were given general information on healthy food choices. Both groups were instructed on increasing physical activity. There was no significant difference in baseline IIEF-5 scores (14.4±3.8 vs. 14.9±3.7). Two years after randomization, 13/35 men in the intervention group and 2/30 in the control group had restoration of normal erectile function with IIEF-5 ≥22 (P=0.015). Nutrient intake, endothelial function scores and CRP were independent predicators of IIEF-5 score (41). Of note, physical activity was increased in both groups, with no significant difference between them.
While the generalizability of these results may be problematic given the exclusion of men with comorbidities typically accompanying ED (CVD, diabetes and hypertension), the Mediterranean diet adopted in this study reflects many of the dietary recommendations outlined by the American College of Cardiology/American Heart Association (ACC/AHA) Task Force (42). This panel made grade A recommendations for the “MED pattern” diet which is similarly high in fruits, vegetables, whole grains, fish, polyunsaturated fats, nuts, and lower in unsaturated fats, red meat, sugar-sweetened beverages. These recommendations can provide a framework for counseling patients with ED and obesity about dietary modification.
Physical activity
Along with its implications in CVD, sedentary lifestyle and low rates of physical activity (PhA) have been demonstrated to be significant and independent risk factors for ED (7,43,44). In the Health Professionals Follow-up Survey, PhA measured by metabolic equivalents (METs) was found to be inversely related to risk of ED (8). Patients who performed 16.6 METs per week (equivalent to running ~1.5 h per week or 3 h of rigorous outdoor physical labor) had a 0.8 relative risk of ED in comparison to men expending less than 2.7 METs per week. Increasing activity to 32.6 METs per week reduced the relative risk to 0.7 (8). In another large-scale study, greater degree of sedentary behavior was strongly associated with ED after multivariable adjustment. Men who spent ≥5 h per day using TV/video or computers were almost three times more likely to report ED than men spending <1 h performing the same activities (OR =2.94; 95% CI, 1.56–5.44) (7).
Additionally, there are several prospective trials which investigated alterations in sexual function seen with increased PhA. In a randomized trial of obese but otherwise healthy males undergoing lifestyle modification with intensive diet and exercise counseling, Esposito et al. showed that increased PhA level was an independent predictor of IIEF score, regardless of change in BMI (38). In another prospective, randomized study, La Vignera et al. investigated the role of PhA in men with arterial ED confirmed by Doppler-ultrasound [cavernosal artery PSV <30 cm/s after intracavernosal injection of alprostadil (20 g)] (45). Fifty men were assigned to a protocol of PhA (150 min of moderate intensity aerobic activity per week) plus Mediterranean diet. The control group (n=20) had similar baseline PSV, IIEF, BMI and abided by the Mediterranean diet, without exercise. After three months of intervention, men assigned to PhA had a mean improvement in IIEF to 16.5±1.0 from 11.0±1.0 (P<0.05), while there was negligible change (11.0±0.7 from 10.5±0.7) in the control group. There was also a significant difference in post-intervention IIEF scores between groups (P<0.05). The authors proposed the underlying mechanism of erectile function recovery to be an improvement in endothelial integrity. Serum endothelial precursor cells (EPCs) and endothelial microparticles (EMPs), both markers of endothelial dysfunction, were significantly reduced from baseline after the 3-month period increased PhA.
Similar results were found in investigations of increased PhA in men with multiple medical comorbidities. In a prospective trial of 59 men with chronic and stable heart failure (HF), patients were randomized to supervised cycle ergometer exercise training at moderate intensity (60% of peak VO2 or peak oxygen consumption) for 60 min, 3 times a week, for 8 weeks. The control group did not receive supervised exercise. QOL and sexual activity profile (SAP) assessments (Domain 1, relationship with partner; Domain 2, quality of penile erection; Domain 3, personal wellness) were obtained at baseline and at 8 weeks. In this cohort of men with HF, exercised patients had a significant increase in peak VO2 of 18% (P<0.005), while there was no change in control patients (46). From a psychosocial standpoint, exercised patients had a significant increase in QOL (r=0.80, P<0.001) and an improvement in all domains of the SAP assessment (3.49±3.4 vs. 6.17±3.2, P<0.001). Of note, partner SAP scores also improved significantly (2.47±2.7 vs. 4.87±2.5, P<0.001). This study points to global improvement in sexual function and well-being for both the exercised patient and his partner.
Despite the well-supported concept that physical activity enhances erectile function and well-being, there remains a paucity of literature on the quantity of PhA needed establish a clinical improvement in erectile function. The ACC/AHA Task Force published Grade B recommendations for PhA for reduction in hypertension, LDL-C and non-LDL-C which can been suggested as guidelines for men with ED (42). Recommendations include 3–4 sessions of moderate-to-vigorous intensity physical activity lasting 40 minutes per session.
Alternatively, some have recommended achieving a minimum kcal expenditure per week given findings of a relationship between IIEF-5 score and Paffenbarger score (PhA in kilocals per week) up to 4,000 kcal/week (47). In a multiple regression analysis, Kratzik et al. (47) demonstrated that after adjusting for total and bioavailable testosterone, age, BMI, smoking status, number of cigarettes, CVD, and hypertension, higher PhA significantly reduced the risk of ED. PhA >3,000 kcal/week significantly reduced the likelihood of severe ED (IIEF-5 <8) by 82.9% (P=0.018). With growing popularity of wearable activity trackers and mobile applications, patients can be encouraged to increase PhA through self-monitoring per ACC/AHA recommendations or through minimum kcal expenditure.
Alcohol and illicit drugs
The role of alcohol and illicit drugs in the progression and treatment of ED is decidedly less clear. In the initial cross-sectional data from the MMAS, higher rates of ED were associated with consumption of copious amounts of alcohol (>600 mL/week) (6). Furthermore, in a large, multi-national epidemiologic study, heavy and no alcohol consumption were associated with higher risk of ED as compared to moderate alcohol intake (1 to 7 drinks per week), though not significantly (48). On the contrary, in the HPFS study, there was no change in relative risk of ED across all categories of alcohol consumption (8).
In the rat model, chronic alcohol consumption leads to an upregulation of endothelin-1 (ET-1) which acts as a vasoconstrictor in the corpora cavernosa (CC). Following electrical stimulation of the major pelvic ganglion, ethanol treated rats demonstrated significantly reduced erectile response as measured by maximal intracavernosal pressure/mean arterial pressure (ICP/MAP) (49). These results provide some basis for investigation in human subjects. Whether changes in CC ET-1 levels are sustained after ethanol cessation warrants investigation.
Illicit drug use was studied in a cross-sectional trial of Taiwanese detainees (N=701, mean age 33.8 years) with a history of drug abuse versus controls (N=196) (50). Heroin, amphetamine and MDMA (“ecstasy”) were the most commonly reported drugs of abuse in this detainee population. Over one third (36.4%) of drug abusers were found to have ED as reported by IIEF-5 score, with 10% reporting severe ED. Drug abusers were found to have significantly lower mean IIEF scores in each domain as compared to controls. Additionally, multiple logistic regression analysis proved dosing frequency to be a predictor of ED. Men who reported use of illicit substances ≥3 times per day had significantly increased likelihood of ED compared to men using <1 time per day (OR =1.6; 95% CI, 1.11–2.46). Duration of abuse was not found to be a predictor of ED.
Amphetamine, a commonly abused CNS stimulant with purported aphrodisiac effects, was studied in a cross-sectional study of 1,159 mono-users (mean age 31.9±7.5 years) compared to age-matched controls (51). Prevalence of ED was significantly higher in amphetamine abusers than in controls (29.3% vs. 11.9%, P<0.001). After adjusting for age, BMI, marital status, diabetes, hypertension, depression, smoking, and alcoholism, drug users were still two times more likely to have ED than controls (OR =2.1; 95% CI, 1.2–3.6). Three domains of sexual function in particular were found to be significantly lower in drug abusers than controls: erectile function, orgasmic function, overall satisfaction. Intercourse satisfaction and sexual desire did not appear to be affected by amphetamine use.
The impact of cannabis use on sexual function has been studied in vitro and in vivo, and remains incompletely understood. Shamloul and Bella thoroughly reviewed the paradoxical impact of cannabis in male sexual health (52). The authors highlighted studies of cavernosal and central nervous system cannabinoid (CB) receptors, which were seen to have a conflicting effect on erectile function. Human clinical trials have also suggested varying effects of cannabis on erectile function, sexual pleasure and satisfaction, duration of intercourse and desire. Further studies utilizing validated instruments are certainly warranted given the expanding use and decriminalization of cannabis in the United States and worldwide.
Importantly, given the cross-sectional nature of studies on alcohol and drug abuse, cause-effect relationships cannot be evaluated. Additionally, psychosocial status of drug and alcohol abusers cannot be controlled for and may have confounding effect on sexual function.
Stress
The cyclical relationship between ED and anxiety, depression, chronic stress, and impaired QoL may seem difficult to approach, but is another potential site of behavioral modification and treatment for ED (53,54). Schmidt et al. performed a meta-analysis of eight clinical trials comparing PDE5-I therapy combined with psychological intervention (i.e., sex therapy, couples counseling, sex education) versus either intervention alone (55). The authors found significant benefit to combined therapy in restoration of erectile function, concluding that addition of psychological intervention, in various formats, resulted in an additional improvement in IIEF when combined with pharmacologic intervention.
In a prospective pilot study of 31 men with newly diagnosed ED, a program of stress management plus tadalafil (N=19) was compared to tadalafil alone (N=12) (56). The stress management program consisted of 8 weeks of diaphragmatic breathing exercises and progressive muscle relaxation with audio CD. Morning salivary cortisol levels were measured at baseline and at the end of intervention. After 8 weeks of intervention, sexual desire scores were found to be negatively correlated with morning salivary cortisol, suggesting high levels of morning cortisol may inhibit sexual drive.
In evaluating and treating patients with ED, it is important to note the patients complete biopsychosocial profile and recognize that anxiety, depression, stress and emotional well-being may not only result from ED, but also contribute to it. Combining PDE5-I with adjunctive modalities aimed at reducing psychological contributors to ED is an important step in lifestyle modification.
Conclusions
Current clinical guidelines for sexual dysfunction assert the foundational role of lifestyle modification in treatment of ED (24). Improvement in erectile function represents a tangible motivator for patients, placing urologists in a unique role as facilitators of preventative strategies that have much broader health implications, including improving cardiovascular, psychological and overall well-being.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rosenthal MB, Berndt ER, Donohue JM, et al. Promotion of prescription drugs to consumers. N Engl J Med 2002;346:498-505. [Crossref] [PubMed]
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999;84:50-6. [Crossref] [PubMed]
- Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med 2007;357:673-81. [Crossref] [PubMed]
- Latini DM, Penson DF, Wallace KL, et al. Longitudinal differences in psychological outcomes for men with erectile dysfunction: results from ExCEED. J Sex Med 2006;3:1068-76. [Crossref] [PubMed]
- McCabe MP, Althof SE. A systematic review of the psychosocial outcomes associated with erectile dysfunction: does the impact of erectile dysfunction extend beyond a man's inability to have sex? J Sex Med 2014;11:347-63. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [PubMed]
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007;120:151-7. [Crossref] [PubMed]
- Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med 2003;139:161-8. [Crossref] [PubMed]
- Kupelian V, Araujo AB, Chiu GR, et al. Relative contributions of modifiable risk factors to erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. Prev Med 2010;50:19-25. [Crossref] [PubMed]
- Araujo AB, Hall SA, Ganz P, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol 2010;55:350-6. [Crossref] [PubMed]
- Montorsi F, Briganti A, Salonia A, et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol 2003;44:360-4; discussion 364-5. [Crossref] [PubMed]
- Guay AT. Relation of endothelial cell function to erectile dysfunction: implications for treatment. Am J Cardiol 2005;96:52M-56M. [Crossref] [PubMed]
- Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart 2003;89:251-3. [Crossref] [PubMed]
- Loprinzi PD, Nooe A. Erectile dysfunction and mortality in a national prospective cohort study. J Sex Med 2015;12:2130-3. [Crossref] [PubMed]
- Nehra A, Jackson G, Miner M, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc 2012;87:766-78. [Crossref] [PubMed]
- Shabsigh R, Klein LT, Seidman S, et al. Increased incidence of depressive symptoms in men with erectile dysfunction. Urology 1998;52:848-52. [Crossref] [PubMed]
- Kennedy SH, Dugré H, Defoy I. A multicenter, double-blind, placebo-controlled study of sildenafil citrate in Canadian men with erectile dysfunction and untreated symptoms of depression, in the absence of major depressive disorder. Int Clin Psychopharmacol 2011;26:151-8. [Crossref] [PubMed]
- Fisher WA, Rosen RC, Mollen M, et al. Improving the sexual quality of life of couples affected by erectile dysfunction: a double-blind, randomized, placebo-controlled trial of vardenafil. J Sex Med 2005;2:699-708. [Crossref] [PubMed]
- O'Leary MP, Althof SE, Cappelleri JC, et al. Self-esteem, confidence and relationship satisfaction of men with erectile dysfunction treated with sildenafil citrate: a multicenter, randomized, parallel group, double-blind, placebo controlled study in the United States. J Urol 2006;175:1058-62. [Crossref] [PubMed]
- Rosen R, Shabsigh R, Berber M, et al. Efficacy and tolerability of vardenafil in men with mild depression and erectile dysfunction: the depression-related improvement with vardenafil for erectile response study. Am J Psychiatry 2006;163:79-87. [Crossref] [PubMed]
- Seftel AD, Buvat J, Althof SE, et al. Improvements in confidence, sexual relationship and satisfaction measures: results of a randomized trial of tadalafil 5 mg taken once daily. Int J Impot Res 2009;21:240-8. [Crossref] [PubMed]
- Claes HI, Andrianne R, Opsomer R, et al. The HelpED study: agreement and impact of the erection hardness score on sexual function and psychosocial outcomes in men with erectile dysfunction and their partners. J Sex Med 2012;9:2652-63. [Crossref] [PubMed]
- Gupta BP, Murad MH, Clifton MM, et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med 2011;171:1797-803. [Crossref] [PubMed]
- Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 2010;7:3572-88. [Crossref] [PubMed]
- Kałka D, Domagała Z, Rakowska A, et al. Modifiable risk factors for erectile dysfunction: an assessment of the awareness of such factors in patients suffering from ischaemic heart disease. Int J Impot Res 2016;28:14-9. [Crossref] [PubMed]
- Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med 2000;30:328-38. [Crossref] [PubMed]
- Cao S, Yin X, Wang Y, et al. Smoking and risk of erectile dysfunction: systematic review of observational studies with meta-analysis. PLoS One 2013;8:e60443. [Crossref] [PubMed]
- Kupelian V, Link CL, McKinlay JB. Association between smoking, passive smoking, and erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. Eur Urol 2007;52:416-22. [Crossref] [PubMed]
- Cao S, Gan Y, Dong X, et al. Association of quantity and duration of smoking with erectile dysfunction: a dose-response meta-analysis. J Sex Med 2014;11:2376-84. [Crossref] [PubMed]
- Hirshkowitz M, Karacan I, Howell JW, et al. Nocturnal penile tumescence in cigarette smokers with erectile dysfunction. Urology 1992;39:101-7. [Crossref] [PubMed]
- Huang YC, Chin CC, Chen CS, et al. Chronic cigarette smoking impairs erectile function through increased oxidative stress and apoptosis, decreased nNOS, endothelial and smooth muscle contents in a rat model. PLoS One 2015;10:e0140728. [Crossref] [PubMed]
- Pourmand G, Alidaee MR, Rasuli S, et al. Do cigarette smokers with erectile dysfunction benefit from stopping?: a prospective study. BJU Int 2004;94:1310-3. [Crossref] [PubMed]
- Chan SS, Leung DY, Abdullah AS, et al. Smoking-cessation and adherence intervention among Chinese patients with erectile dysfunction. Am J Prev Med 2010;39:251-8. [Crossref] [PubMed]
- Sighinolfi MC, Mofferdin A, De Stefani S, et al. Immediate improvement in penile hemodynamics after cessation of smoking: previous results. Urology 2007;69:163-5. [Crossref] [PubMed]
- Harte CB, Meston CM. Association between smoking cessation and sexual health in men. BJU Int 2012;109:888-96. [Crossref] [PubMed]
- Schwartz JL. Methods of smoking cessation. Med Clin North Am 1992;76:451-76. [PubMed]
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008;35:158-76. [PubMed]
- Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004;291:2978-84. [Crossref] [PubMed]
- Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972-8. [Crossref] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. [Crossref] [PubMed]
- Esposito K, Ciotola M, Giugliano F, et al. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res 2006;18:405-10. [Crossref] [PubMed]
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2960-84. [Crossref] [PubMed]
- Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 2000;56:302-6. [Crossref] [PubMed]
- Nicolosi A, Glasser DB, Moreira ED, et al. Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: a population study. Int J Impot Res 2003;15:253-7. [Crossref] [PubMed]
- La Vignera S, Condorelli R, Vicari E, et al. Aerobic physical activity improves endothelial function in the middle-aged patients with erectile dysfunction. Aging Male 2011;14:265-72. [Crossref] [PubMed]
- Belardinelli R, Lacalaprice F, Faccenda E, et al. Effects of short-term moderate exercise training on sexual function in male patients with chronic stable heart failure. Int J Cardiol 2005;101:83-90. [Crossref] [PubMed]
- Kratzik CW, Lackner JE, Märk I, et al. How much physical activity is needed to maintain erectile function? Results of the Androx Vienna Municipality Study. Eur Urol 2009;55:509-16. [Crossref] [PubMed]
- Nicolosi A, Moreira ED Jr, Shirai M, et al. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology 2003;61:201-6. [Crossref] [PubMed]
- Muniz JJ, Leite LN, De Martinis BS, et al. Chronic ethanol consumption induces erectile dysfunction: role of oxidative stress. Life Sci 2015;141:44-53. [Crossref] [PubMed]
- Bang-Ping J. Sexual dysfunction in men who abuse illicit drugs: a preliminary report. J Sex Med 2009;6:1072-80. [Crossref] [PubMed]
- Chou NH, Huang YJ, Jiann BP. The impact of illicit use of amphetamine on male sexual functions. J Sex Med 2015;12:1694-702. [Crossref] [PubMed]
- Shamloul R, Bella AJ. Impact of cannabis use on male sexual health. J Sex Med 2011;8:971-5. [Crossref] [PubMed]
- Perelman MA. Psychosocial evaluation and combination treatment of men with erectile dysfunction. Urol Clin North Am 2005;32:431-45. vi. [Crossref] [PubMed]
- Hedon F. Anxiety and erectile dysfunction: a global approach to ED enhances results and quality of life. Int J Impot Res 2003;15:S16-9. [Crossref] [PubMed]
- Schmidt HM, Munder T, Gerger H, et al. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: a narrative review and meta-analysis. J Sex Med 2014;11:1376-91. [Crossref] [PubMed]
- Kalaitzidou I, Venetikou MS, Konstadinidis K, et al. Stress management and erectile dysfunction: a pilot comparative study. Andrologia 2014;46:698-702. [Crossref] [PubMed]