
Outcomes comparison of testicular versus ejaculated sperm for intracytoplasmic sperm injection in infertile men with high DNA fragmentation: updated systematic review and meta-analysis
Highlight box
Key findings
• The sperm DNA fragmentation (SDF) rates are lower in testicular sperm than in ejaculated sperm, intracytoplasmic sperm injection with testicular sperm (Testi-ICSI) is correlated with better clinical outcomes in infertile males with high SDF levels.
What is known and what is new?
• The testicular sperm instead of ejaculated sperm for ICSI in infertile men with high SDF remains controversial.
• Results of this updated meta-analysis reveal that SDF rates are lower in testicular sperm than in ejaculated sperm and that Testi-ICSI is correlated with better clinical outcomes.
What is the implication, and what should change now?
• It will be useful to assess the implications of DNA damage prior to conducting ICSI in clinical recommendations. However, treatment with Testi-ICSI should be recommended for groups of men with high SDF only after several other strategies to correct underlying factors to alleviate SDF have failed.
Introduction
The development of intracytoplasmic sperm injection (ICSI) in 1992 was a major breakthrough in the treatment of infertile men, provided that spermatozoa can be identified either in the ejaculate semen or testicular tissue (1). As the method has improved progressively over recent decades, it has also been demonstrated through clinical data that there are still some limitations that lead to low pregnancy rates for ICSI (2). Recent evidence supports the idea that sperm DNA fragmentation (SDF) may have adverse effects on the outcomes of ICSI (3,4). Several testicular and post-testicular factors are known to explain the etiology of SDF (5).
Previous trials have shown that the source of sperm could possibly influence sperm DNA integrity (6). Given the premise that sperm may suffer oxidative stress and nuclear DNA damage during transit through the male genital tract, the use of testicular sperm for ICSI is a better choice for patients with high DNA fragmentation index (DFI) in ejaculated sperm to improve ICSI outcomes (6,7).
Additionally, numerous clinical studies in recent decades have explored the contribution of DNA integrity to clinical outcomes after ICSI, but the results remain controversial. Some reports concluded that high SDF negatively affects ICSI outcomes, but others have indicated that there are only limited data available showing an association of DNA damage with reproductive outcomes, and have suggested that there are some specific deficiencies in the available evidence supporting this view due to the lack of well-designed, clinical, randomized and controlled studies, and the need for application of standardized test assays and cut-off values for SDF. These results have led to different opinions and a variety of recommendations in clinical practice guidelines (8-11).
Notably, the clinical application of using testicular sperm instead of ejaculated sperm for ICSI in non-azoospermic infertile men with confirmed post-testicular high SDF is still being debated. On the one hand, previous systematic reviews and meta-analyses that have tested the contribution of DNA integrity to ICSI outcomes have not resolved this controversy (3,6,8,12). Some of these meta-analyses are characterized by several limitations, among which were the inclusion of cryptozoospermia cases, as well as studies that analyzed previous ICSI failures without using the DFI, or which were not about ICSI but in vitro fertilization (IVF) outcomes. On the other hand, additional studies that have been made available in scientific databases since those studies were published, and which offer new relevant information on this topic, can now be used to make a comprehensive reassessment of this research question. Herein, the present updated systematic review and meta-analysis aims to collect and summarize the available evidence on whether couples with high levels of DFI will benefit more from intracytoplasmic sperm injection with testicular sperm (Testi-ICSI) as compared to intracytoplasmic sperm injection with ejaculated sperm (Ejac-ICSI), in order to contribute objectively to a consistent clinical recommendation. We present this article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-415/rc).
Methods
Literature search strategy
According to the study protocol, we conducted a systematic search using the electronic databases PubMed, Embase, Web of Science and the Cochrane Central Register of Controlled Trials (CENTRAL), to identify all relevant articles, encompassing studies from the earliest records until 4 May 2022. The search was performed according to the inclusion and exclusion criteria defined in a Population, Intervention, Comparison, Outcomes, Study (PICOS) design structure, as shown in Table S1. The search strategy used different combinations of the following entry terms: “Sperm Injections, Intracytoplasmic”[Mesh], “Injection, Intracytoplasmic Sperm”, “Injections, Intracytoplasmic Sperm”, “Intracytoplasmic Sperm Injection”, “Sperm Injection, Intracytoplasmic”, “Intracytoplasmic Sperm Injections”, “ICSI”, “Injections, Sperm, Intracytoplasmic”, “Sperm Retrieval”[Mesh], “Sperm Retrievals”, “Sperm Aspiration”, “Testicular Sperm Aspiration”, “Sperm Aspiration, Testicular”, “Testicular Sperm Retrieval”, “Sperm Retrieval, Testicular”, “Sperm Aspiration, Vasal”, “Vasal Sperm Retrieval”, “Sperm Retrieval, Vasal”, “Epididymal Sperm Aspiration”, “Sperm Aspiration, Epididymal”, “Epididymal Sperm Retrieval”, “Sperm Retrieval, Epididymal”, “testicular sperm”, “Ejaculation”[Mesh], “Ejaculations”, “ejaculate”, “ejaculated sperm”, “DNA fragmentation index”, “sperm DNA fragmentation”, “high DNA fragmentation”, “high sperm DNA fragmentation”, “sperm DNA damage” and “sperm chromatin integrity OR damage”. Furthermore, we also searched trial registers for ongoing and registered trials, specifically, PROSPERO and ClinicalTrials.gov, using the search terms as follows: “sperm DNA fragmentation”, “sperm DNA damage”, “sperm chromatin integrity OR damage”, “testicular sperm”, “ejaculated sperm”, “intracytoplasmic sperm injection”, with the filter “human” in any language. Additionally, a manual search was performed using reference lists and cited articles in the identified studies, looking for further relevant publications. Only English articles using human subjects were included, and non-comparative studies, meta-analyses and systematic reviews were excluded. There were no other restrictions on the types of studies that were included. Endnote was applied to exclude repeated and unqualified literature.
Eligibility criteria and selection of studies
The literature search was performed by two researchers (G.Z. and Y.Z.) in different stages, as depicted in the flowchart shown in Figure 1. Initially, all results of the searches were exported to EndNote, including the reference, DOI, title, abstract, authors and article type, and at that point duplicates and review articles were removed. We then used a two-stage approach in screening for study inclusion to perform meta-analysis: first, titles and abstracts were screened, excluding articles that did not meet the eligibility criteria or that did not include relevant outcomes. Second, the full text of articles identified as meeting the initial screening eligible criteria were retrieved and read in detail. Two researchers (G.Z. and Y.Z.) evaluated each title, abstract and full manuscript of the articles, extracted and entered the data into two separate databases, and any disagreement was resolved by consensus with the opinion of a third researcher (D.L.). Another researcher (H.B.) re-examined the full manuscripts of all included studies to ensure accuracy of data collection and entry. When studies did not have all the necessary information, we contacted the corresponding authors to request the missing data. These studies were excluded if no data were provided or no response was received by the time of meta-analysis.
The several eligibility criteria used in the current meta-analysis were defined in the PICOS design (showed in Table S1) as follows: (I) randomized controlled trial (RCT) and prospective or retrospective non-randomized observational studies that enrolled human participants were included if they evaluated the effects of using Testi-ICSI and Ejac-ICSI among couples with high SDF in the ejaculate semen, with or without SDF test in the testicular sperm; (II) all patients treated with ICSI, in combination with screening for SDF; (III) available data on SDF in the ejaculate semen or testicular sperm, and primary pregnancy outcomes including live birth rate (LBR) (per cycle), fertilization rate (FR), clinical pregnancy rate (CPR), miscarriage rate (MR) and LBR. The exclusion criteria included the following: (I) full text could not be obtained; (II) no control was used in the study; (III) diagnosis of azoospermia or cryptozoospermia, or severe oligozoospermia (<5×106/mL); (IV) studies comparing the use of Testi-ICSI and Ejac-ICSI in which the SDF levels were not examined in semen; (V) reviews, letters, conference papers, case reports, case analyses, animal experiments and commentary articles, or previous systematic reviews and meta-analyses. Additionally, studies performing ICSI with an additional non-conventional sperm selection were also excluded. It is worth noting that the studies on the SDF measurement of ejaculated sperm are more, but on the SDF test of testicular sperm are relatively fewer. The relatively older researches provided important data on SDF test in the testicular sperm, these older articles were also included in this meta-analysis.
Outcomes measures
The primary outcomes of interest in the current meta-analysis were paired SDF levels between ejaculated and testicular sperm, and the achievement of pregnancy per patient expressed as either CPRs [calculated as the number of clinical pregnancies per fresh embryo transfers (ETs)] or LBRs. Clinical pregnancy was defined as a pregnancy with observed evidence of intrauterine sac, such as detection of a gestational sac by ultrasound visualization with fetal heart activity or other definitive clinical signs at 6–8 weeks of gestation. Secondary outcomes of interest that were evaluated were FRs and MRs. The definition of fertilization included normal fertilization, abnormal fertilization and delayed fertilization. Miscarriage included any identified clinical pregnancy that resulted in fetal loss before the 20th week of gestation. The LBR was defined as the ratio between the number of deliveries of one or more living infants and the number of fresh ETs.
Data extraction
Data extraction was performed independently from the final list of the eligible articles by two authors (G.Z. and Y.Z.), and any disagreement between the authors responsible for data extraction was resolved by consensus with a third reviewer (D.L.). The data recorded for study characteristics were as follows: the first author, year of publication, study design, the mean age of the patients, the number of patients, the type of test assays and cut-off value used to assess SDF, pregnancy outcomes (FRs, CPRs, MRs and LBRs) after ICSI, and inclusion and exclusion criteria. All key extracted data relating to clinical pregnancy outcomes in the Testi-ICSI and Ejac-ICSI among couples with high SDF in the ejaculate semen were extracted and used in the statistical analyses described below.
Statistical analysis
Statistical analyses were conducted with the use of Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). For dichotomous data from the eligible studies, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated, whereas for continuous outcomes pooled with the use of an inverse variance model, the standardized mean difference (SMD) and 95% CI were used. A Chi-squared test and the I2 index were utilized to evaluate statistical heterogeneity among different trials. We assumed that heterogeneity was significant when the P value by χ2 test was <0.05, or the I2 was ≥50%. If I2≥50% and P<0.05, the results of the random effects model (REM) were provided; otherwise, the fixed effect model (FEM) was applied (13). Unless specified otherwise, a P value (two-sided) of <0.05 was considered statistically significant for all comparisons. We performed post hoc subgroup analyses to investigate potential sources of the heterogeneity and how possible differences were influenced among these methods considering the type of the SDF assay as a subgroup variable.
Sensitivity analysis and risk of bias assessment
Furthermore, funnel plot asymmetry tests were used to qualitatively assess publication bias, and the leave-one-out approach and recalculating summary ORs were conducted for sensitivity analyses, both using Review Manager 5.3 to verify the leverage of individual studies on the pooled results. When individual studies were removed in turn, no significant change in the summarized conclusion signified that the results were reliable. As no eligible randomized studies were found during this search, the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool, with questions covering seven domains of bias, was used to evaluate the risks of bias in the included literature (14).
Certainty of evidence
Certainty of the overall evidence for each outcome was assessed as very low, low, moderate, or high, based on the assessment of the domains for risk of bias, inconsistency, indirectness, publication bias, intransitivity, incoherence, and imprecision, by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework (15). The quality of evidence from all non-randomized included literature was initially categorized as low, then upgraded for large effect sizes or dose-response gradient or attenuation of the pooled risk estimates by plausible confounders, and downgraded based on risk of bias or inconsistency or indirectness or imprecision or publication bias.
Results
Identification and selection of articles in the meta-analysis
Inclusion of studies for qualitative analysis
The details of the article identification and selection process used in the meta-analysis are shown in Figure 1. The search strategy initially yielded 239 records, of which 71 were excluded as duplicates. Out of the 166 articles screened, 144 studies were excluded after preliminary screening for irrelevance to the inclusion criteria based on the title or abstract. This resulted in 22 articles being deemed eligible for further consideration, which were then downloaded and subjected to full-text assessment. Among these, 11 papers were rejected after carefully reading the full text because of exclusion criteria: eight articles having subjects with severe oligozoospermia, two articles having subjects with cryptozoospermia and one study on couples with poor ovarian response. Thus, data from a total of 11 published retrospective-cohort or prospective-cohort studies were included in this systematic review.
Basic information about the included studies
Table 1 shows the basic characteristics of all the studies which fulfilled the inclusion criteria for meta-analysis (16-26). Among the 11 selected articles, there were a total of 1,114 cases with high levels of SDF in neat semen, including 505 cases for the Testi-ICSI group and 609 cases for the Ejac-ICSI group. The number of studies in each meta-analysis varied depending on the parameters reported, ranging from two to five. Notably, high levels of SDF were defined using varying parameters for thresholds, and different test methods were used in the 11 articles selected for data analysis. Four studies (22-25) provided paired data on SDF between ejaculated and testicular sperm, involving 119 patients who served as their own controls. In these four studies, the SDF thresholds were 30% using the sperm chromatin dispersion (SCD) test as the assay for SDF assessment [one study (22)]; SDF ≥15% using the terminal deoxyribonucleotide transferase-mediated dUTP nick-labeling (TUNEL) assay [one study (25)]; and SDF ≥30% using the TUNEL assay [two studies (23,24)]. Among the other seven studies, there were two subgroups assessed by sperm chromatin structure assay (SCSA) with SDF ≥30% or SDF <30% in one study (17), two subgroups assessed respectively by SCSA (% DFI) ≥25% or TUNEL (% DFI) ≥36% in one study (18), SCSA (% DFI) ≥30% assessed in one study (17), the SDF ≥30% with SCD used in one study (19), the SDF ≥15% with TUNEL assessed in one study (20), the SDF ≥29% with sperm chromatin integrity test (SCIT) used in one study (21), and the alkaline comet assay used in one study (26). Regarding the pregnancy outcomes (FRs, CPRs, MRs and LBRs) after ICSI in the 11 articles that fulfilled the inclusion criteria for meta-analysis, nine studies (851 cycles) provided sufficient data on CPRs, six studies (5,762 cycles) had data on FRs, seven studies (705 cycles) reported LBRs, and seven studies (244 cycles) presented data on the MR. Cycles with failed embryo development, blastocysts frozen and number of metaphase II (M II) oocytes retrieved were inconsistently reported in all the included studies and therefore did not allow estimation of weighted mean difference (WMD) for comparison.
Table 1
| No. | Study | Study design | Patients (n) | Male age (years), mean ± standard deviation/range | SDF methodology | Cut-off value | SDF results between testicular and ejaculated sperm | Sperm retrieval complication | Fertilization rates | Clinical pregnancy rates | Miscarriage rates | Live birth rates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alharbi et al. 2020, (16) | Cohort study, retrospective | T-ICSI: 52, E-ICSI: 87 | T-ICSI: 38.9±5.9, E-ICSI: 37.0±6.6 | SCSA | 30% | NR | No early complications | T-ICSI: 58.02%, E-ICSI: 60.73% (P=0.03) | T-ICSI: 48.6%, E-ICSI: 38.7% (P=0.41) | T-ICSI: 11.1%, E-ICSI: 16.7% (P=0.66) | T-ICSI: 36.4%, E-ICSI: 30.0% (P=0.59) |
| 2 | Zhang et al. 2019, (17) | Cohort study, retrospective | T-ICSI: 61, E-ICSI: 41 | T-ICSI: 33.5±4.1, E-ICSI: 34.6±4.5 | SCSA | 30% | NR | None | NR | T-ICSI: 36%, E-ICSI: 14.6% (P=0.017) | T-ICSI: 0%, E-ICSI: 3.3% (P<0.159) | T-ICSI: 41%, E-ICSI: 9.8% (P<0.001) |
| 3 | Herrero et al. 2019, (18) | Cohort study, retrospective | T-ICSI: 77, E-ICSI: 68 | T-ICSI: 40.5±6.2, E-ICSI: 40.1±5.5 | TUNEL/SCSA | 36% (TUNEL), 25% (SCSA) | NR | NR | NR | T-ICSI: 31.2%, E-ICSI: 19.7% (P=NS) | T-ICSI: 25%, E-ICSI: 41.7% (P<0.05) | T-ICSI: 23.4%, E-ICSI: 11.4% (P<0.05) |
| 4 | Arafa et al. 2018, (19) | Cohort study, prospective | 36 | T-ICSI: 38.42±12.2, E-ICSI: 37.53±11.4 | SCD | 30% | NR | NR | T-ICSI: 47.8%, E-ICSI: 46.4% (P=0.155) | T-ICSI: 38.89%, E-ICSI: 13.5% (P<0.0001) | NR | T-ICSI: 38.89%, E-ICSI: 8.33% (P<0.0001) |
| 5 | Pabuccu et al. 2017, (20) | Cohort study, retrospective | T-ICSI: 31, E-ICSI: 40 | T-ICSI: 33.0±3.9, E-ICSI: 33.9±3.7 | TUNEL | 30% | NR | None | T-ICSI: 74.2%, E-ICSI: 70.9% (P=0.619) | T-ICSI: 41.9%, E-ICSI: 20% (P=0.045) | T-ICSI: 3.2%, E-ICSI: 17.5% (P=NR) | T-ICSI: 38.7%, E-ICSI: 7.1% (P=NR) |
| 6 | Bradley et al. 2016, (21) | Cohort study, retrospective | T-ICSI: 80, E-ICSI: 1,727 | T-ICSI: 34.9–41.1, E-ICSI: 33.3–40.1 | SCIT | 29% | NR | NR | T-ICSI: 57.0%, E-ICSI: 66.0% (P<0.001) | T-ICSI: 53.0%, E-ICSI: 26.2% (P<0.05) | T-ICSI: 11.4%, E-ICSI: 9.1% (P<0.05) | T-ICSI: 49.8%, E-ICSI: 24.2% (P=0.020) |
| 7 | Esteves et al. 2015, (22) | Cohort study, prospective | T-ICSI: 81, E-ICSI: 91 | T-ICSI: 37.3±4.6, E-ICSI: 36.8±3.1 | SCD | 30% | Yes; T-ICSI: 8.3%, E-ICSI: 40.7% | 6.2% complication rate (4 patients of pain, 2 patients of moderate scrotal swelling) | T-ICSI: 56.1%, E-ICSI: 69.4% (P=0.0001) | T-ICSI: 51.9%, E-ICSI: 40.2% (P=0.131) | T-ICSI: 46.7%, E-ICSI: 26.4% (P=0.007) | T-ICSI: 10.0%, E-ICSI: 34.3% (P=0.012) |
| 8 | Moskovtsev et al. 2012, (23) | Cohort study, prospective | T-ICSI: 8, E-ICSI: 10 | T-ICSI: 38.8±4.1, E-ICSI: 36.5±2.3 | TUNEL | 30% | Yes*; T-ICSI: 14.9%±5.0%, E-ICSI: 40.6%±14.8% (P<0.05) | None | NR | NR | NR | NR |
| 9 | Moskovtsev et al. 2010, (24) | Cohort study, prospective | 12 | 43.9± 9.7 (33.8–64.9) | TUNEL | 30% | Yes; T-ICSI: 39.7%±14.8%, E-ICSI: 13.3%±7.3% (P<0.001) | None | NR | NR | NR | NR |
| 10 | Greco et al. 2005, (25) | Cohort study, retrospective | 18 | 28–55 | TUNEL | 15% | Yes | NR | T-ICSI: 74.9%, E-ICSI: 70.8% (P>0.05) | T-ICSI: 44.4%, E-ICSI: 5.6% (P<0.05) | T-ICSI: 0/8, E-ICSI: 1/1 (P=NR) | T-ICSI: 8/8, E-ICSI: 0/1 (P=NR) |
| 11 | Lewis et al. 2004, (26) | Cohort study, retrospective | 28 | NR | The alkaline comet assay | NR | NR | NR | NE | T-ICSI: 50.0%, E-ICSI: 38.9% (P<0.05) | NR | NR |
*, aneuploidy rates (T-ICSI vs. E-ICSI: 12.41%±3.7% vs. 5.77%±1.2%, P<0.05). ICSI, intracytoplasmic sperm injection; SDF, sperm DNA fragmentation; T-ICSI, intracytoplasmic sperm injection with testicular sperm; E-ICSI, intracytoplasmic sperm injection with ejaculated sperm; SCSA, sperm chromatin structure assay; NR, not reported; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling; NS, not significant; SCD, sperm chromatin dispersion; SCIT, sperm chromatin integrity test.
Assessment of research quality
Because no eligible randomized studies were found in the included articles, the methodological quality of the trials was evaluated using the ROBINS-I tool. As shown in Table S2, the assessment results of all of the studies indicated that three papers were assessed to be at low risk of bias (high quality), six papers had moderate risk of bias (moderate quality) and two papers were considered at severe or critical risk of bias (low quality).
Meta-analysis: quantitative analysis
A summary of the pooled OR values from each meta-analysis assessing paired data on SDF between ejaculated and testicular sperm, and the respective pregnancy outcomes of Testi-ICSI or Ejac-ICSI data with high levels of SDF considered, including subgroup analyses according to the SDF method used, are provided in Figures 2,3.


Comparing SDF levels between testicular and ejaculated sperm
The results from comparing SDF levels revealed a significant difference association (OR =−25.81; 95% CI: −34.82, −16.81; P<0.00001) between testicular and ejaculated sperm which served as their own controls in four studies (22-25), indicating lower SDF rates in testicular than in ejaculated sperm, with severe heterogeneity among studies (I2=94%, P<0.00001) (Figure 2); thus, the random effect model was applied. Subgroup analysis was performed by the SDF method (TUNEL, SCD) to account for heterogeneity in this case. Performing analysis separately by the methods used for assessing SDF showed that heterogeneity was reduced (I2=43%, P=0.17) in three studies (23-25) with TUNEL assay, revealing that the SDF method used may explain the difference in SDF between studies (Figure 2). During the sensitivity analyses, the magnitude of the pooled effect size was changed and the heterogeneity was reduced by the removal of the studies by Esteves et al. (22) (OR =−21.83; 95% CI: −27.78, −16.37; I2=43%) and Greco et al. (25) (OR =−30.50; 95% CI: −34.76, −26.25; I2=27%), whereas the ORs were always close to unity after removal of any of the other studies (Table S3). Thus, the results indicated that the heterogeneity in the data could be explained by the method of SDF analysis and the type of participants. Additionally, they also indicated the need for further studies to evaluate the magnitude of the observed effect. Due to the number of included trials being too small, no funnel plot was constructed for the assessment of publication bias.
Pregnancy outcomes of Testi-ICSI vs. Ejac-ICSI data with high levels of SDF
Six studies (16,19-22,25) provided fertilization data, with either testicular sperm for 3,128 oocytes injected, or ejaculated sperm for 2,634 oocytes injected, in high-SDF patients. The mean FRs with the use of Testi-ICSI and Ejac-ICSI were 57.7% and 63.4%, respectively, with a trend to lower FRs but no significant difference in the Testi-ICSI group (OR =0.87; 95% CI: 0.67, 1.12; P=0.28). Because, in this case, the heterogeneity was deemed substantial (I2=81%, P<0.0001), the REM was applied. Another subgroup analysis was undertaken among studies of patients using the technique for evaluating sperm DNA damage (SCSA, TUNEL, SCD, SCIT) to assess heterogeneity. In the “TUNEL” subgroup, the FR was 74.5% for Testi-ICSI and 70.9% for Ejac-ICSI (P=0.25), with a pooled OR of 1.20 (95% CI: 0.88, 1.65; I2=0%; P=0.91). In the “SCD” subgroup, FRs for testicular versus ejaculated sperm were 52.9% and 60.9%, respectively (P=0.40), with a pooled OR of 0.77 (95% CI: 0.42, 1.42; I2=93%; P=0.0002). It is noteworthy that there was only one trial each for the SCSA and SCIT subgroups, which perhaps affected the ability of this analysis to detect heterogeneity (shown in Figure 3A). Sensitivity analyses with the leave-one-out approach demonstrated that the observed pooled effect size was not materially affected by the removal of individual studies (OR: 0.82–0.95) (Table S4). Funnel plot asymmetry was not found by visual inspection, which showed no significant publication bias (shown in Figure S1A).
Because of the low heterogeneity in the CPR (I2=0%, P=0.49), LBR (I2=4%, P=0.39) and MR (I2=0%, P=0.44) among these studies, the FEM was used for statistical analysis. As shown in Figure 3B-3D, there was significant difference in these items between the Testi-ICSI group and the Ejac-ICSI group, with combined effects of (OR =2.36; 95% CI: 1.71, 3.24; P<0.00001), (OR =3.10; 95% CI: 2.13, 4.51; P<0.00001), and (OR =0.28; 95% CI: 0.13, 0.60; P=0.001), respectively. Visual inspection of the funnel plots revealed all literature data were symmetrically distributed around the center, as shown in Figure S1B-S1D. Sensitivity analyses demonstrated that removal of any study in turn had no significant effect on the observed pooled effect size (Tables S5-S7). Therefore, sensitivity analyses and publication bias assessment showed that the results were reliable. These results indicated a tendency for sperm DNA damage to decrease CPR, significant association between high DFI and MR, and much higher LBR among the Testi-ICSI couples as compared to the Ejac-ICSI couples with high SDF levels in semen.
Finally, these findings were considered to be either low- or very-low-quality evidence as assessed by the GRADE framework, due to the nature of the observational study designs and potential confounding biases without adjustment for sufficient confounders, as shown in Table S8.
Discussion
The difference level of SDF between testicular and ejaculated sperm
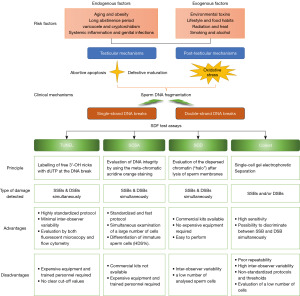
The male germ cells encounter DNA damage deriving from a number of exogenous and endogenous risk factors for infertility insults (27-30) which have different mechanisms during the process of spermatogenesis, including defective chromatin packaging, testicular apoptosis and seminal oxidative stress (shown in Figure 4). Meanwhile, due to a possibly dysfunctional repertoire of repair mechanisms, they do not get correctly repaired in a short time to maintain genomic integrity, which ultimately leads to high SDF (31). Studies have shown that excessive ROS and/or impaired antioxidant defense of the reproductive system production can induce DNA damage, impair genome stability during sperm transport throughout the male genital tract, and potentially impact pregnancy outcomes. Although many therapeutic approaches (32-35) and some advanced sperm selection techniques (36-38) exist to help men with elevated SDF improve their reproductive outcomes. It is worth mentioning that little available evidence, or conflicting evidence, exists to make strong recommendations about the effects of these therapeutic approaches or various sperm selection methods for improving SDF and reproductive outcomes.
Based on the finding that the SDF of testicular sperm is lower than that of ejaculated sperm (6,21,24,25,39), the utilization of testicular spermatozoa has been recommended as a viable strategy for ICSI candidates with extremely high DFI (DFI ≥30%) in their ejaculates to overcome the oxidation-induced damage to sperm DNA integrity and ultimately gain better ICSI outcomes (21,22,40,41). However, the results of a prospective study showed no benefit of testicular sperm for improving reproductive outcomes compared to ejaculated sperm in infertile men with elevated SDF and previous ICSI failure (16). However, the poor quality of the evidence, with no valid SDF testing for testicular sperm (32), and the risk of anesthesia and complications connected with surgical sperm retrieval, as well as the possible higher aneuploidy rate in testicular sperm, are concerns (42,43). Only six reports in the included studies reported complications data, and it was either nonexistent or minor (shown in Table 1). Therefore, the usefulness of testicular sperm in preference over ejaculated sperm in nonazoospermic patients with high SDF undergoing ICSI to improve reproductive outcomes continues to be debated, and new research data have been updated.
At present, Testi-ICSI may overcome infertility in men with confirmed post-testicular SDF, but not among those with cryptozoospermia or untested SDF (44). Considering that higher DFI values were found to be strongly associated with poor sperm quality (45,46), due to intratesticular apoptosis induced by impairment in sperm maturation that leads to early DNA damage, this meta-analysis excluded the literature studying the use of testicular versus ejaculated sperm for ICSI among men with cryptozoospermia or severe oligozoospermia. On the other hand, there are relatively few studies which directly compare the SDF level between testicular sperm and semen spermatozoa, probably because of the technical difficulties involved in performing the test on such low numbers of sperm or on testicular sperm. A previous meta-analysis (6), based on five studies, concluded that there is a lower level of SDF in testicular sperm than in ejaculated sperm, but the 95% CIs were relatively wide and the level of heterogeneity was high (−32.53 to −16.64, I2=92%, respectively). Notably, only four studies (22-25) included in the present meta-analysis provided data comparing paired SDF testing results from ejaculated and testicular sperm and indicating lower SDF rates in testicular sperm than in ejaculated sperm, with severe heterogeneity. Note that the study conducted by Mehta et al. (41) was excluded from our meta-analysis due to their use of testicular sperm from men with severe oligospermia.
The debate about SDF testing methods and thresholds
The integrity of sperm DNA is critical for both natural and assisted fertility outcomes. The standardization of the techniques used for evaluation of single-strand DNA break (SSB) and double-strand DNA break (DSB) may have significant implications for the future management of infertile men with high SDF (47,48). The debate about the evaluation of SDF starts with the DNA analysis techniques, all based on different mechanisms for the detection of DNA breaks. The guidelines of professional societies and the sixth edition of the World Health Organization (WHO) manual provide the detailed procedures of the four commonly used assays for SDF: TUNEL, SCSA, SCD test and single-cell gel electrophoresis assay (comet assay) (49). Unlike TUNEL and SCSA, although SCD and the comet assay can be performed in a private clinic or office setting using commercially available assay kits, due to not needing any advanced equipment, they analyze only a small number of spermatozoa manually under the microscope, which may produce interobserver variability (50). Among the four currently available methods for determining sperm DNA integrity, although TUNEL, SCSA and SCD techniques may potentially detect extensive double-strand breaks, the two-tailed comet assay is the only technique that is able to distinguish between SSBs and DSBs, depending on the methodology (51,52). It is worth mentioning that precise diagnosis and management of SDF thresholds for the prediction of ICSI-related pregnancy are lacking so far. The lack of unanimous consensus on a specific cut-off value of SDF may be attributed to the potential intrinsic and extrinsic factors (53). Although several cut-off values are reported in several recent meta-analyses and studies as having a fair to good overall accuracy in predicting various outcome measures (50,53-55), the heterogeneity of SDF testing methods and thresholds in the published studies present challenges related to the identification of unbiased SDF cut-off values for the prediction of ICSI outcomes. Indeed, there is currently no best investigation technique or optimal cut-offs, and some researchers have concluded a review of all the limitations and advantages of the different methods without expressing a preference for any of them (50,53,56,57) (shown in Figure 4). Consequently, the current SDF testing methods have limited ability to assess the value of SDF in predicting the ICSI outcomes. Notably, varied parameters for thresholds and different test methods were adopted to assess sperm chromatin integrity in the 11 articles selected for data analysis in our meta-analysis, using SCSA, SCD, TUNEL, SCIT or the alkaline comet assay (shown in Table 1). Formal, globally accepted guidelines regarding the choice of SDF assays with strong predictive value for ICSI outcomes are warranted to alleviate the existing discrepancies (32). However, the sixth edition of the WHO manual did not resolve the standardization of SDF assessment, and also stated that the choice of SDF assay, and the diagnostic thresholds used for these assays, depends on laboratory operational procedures and clinical experience, trained personnel, and other factors including cost and run-time in individual clinics (49).
Potential underlying mechanisms for the effect of SDF on ICSI outcomes
The different types of sperm DNA breaks related to ICSI outcomes
Some trials have described SSBs as being related to oxidative stress and leading to low CPRs or prolonged conception time, and that DSBs may be associated with a lack of DNA repair in meiosis and cause a higher risk of miscarriage, low embryo quality and higher risk of implantation failure in ICSI cycles (5,58-60). Some studies have reported that SSBs of DNA fragmentation do not significantly impact embryo development or implantation rates, due to replication still usually being possible in the case of SSBs using the other unaffected strand (50,61). Nonetheless, higher levels of SSBs negatively affect the natural pregnancy outcome (5). Many data have shown that delayed embryo development to blastocyst, which may contribute to a higher implantation failure risk and recurrent first-trimester miscarriage, is observed in patients with high DSB who undergo ICSI treatments and produce embryos (61-64). Although SSBs and DSBs may have different underlying molecular mechanisms for the effect of SDF on ICSI outcomes, most studies do not distinguish between the types of sperm DNA damage attributed to most SDF assays, making it difficult to distinguish between SSBs and DSBs (61). Compared with TUNEL, SCSA and SCD tests, numerous cohort studies have shown that the alkaline comet assay is the most sensitive technique to identify a strong inverse relation between extensive SSBs and the achievement of pregnancy (51,52,54,58). In addition, controversial reproductive outcomes have been observed in different studies on conventional ICSI sperm selection (64-67), and some of them provided the contributing predictive value of oxidative DNA damage for ICSI outcomes (61,68).
Clinical considerations regarding the effect of SDF on ICSI outcomes
Although sperm with high SDF may fertilize an egg with an efficiency similar to that of sperm without DNA fragmentation, the negative effects of paternal chromatin damage often result in impaired embryo development and early abortion (69,70). The results of two studies showed that increasing DFI levels in ICSI reduced FRs (71,72), although other studies have shown no differences (69,70,73-75). Our results suggested that there is no significant tendency to lower FRs with Testi-ICSI compared to Ejac-ICSI. It is significant that the fewer high-quality oocytes and lower FRs might jeopardize the chances of ET, but there is a relative lack of information on this issue provided in this research.
During the early stages of embryonic development, the fragmented male genome results in developing embryonic cells not containing identical and balanced genotypes, a phenomenon known as chaotic mosaicism and considered to be a major cause of miscarriage. In addition, the abnormal embryos with chromosomal abnormalities caused by damaged sperm progressing to live birth may result in congenital malformations in the offspring (76). A large number of studies and meta-analyses have revealed a significant association between recurrent pregnancy miscarriage and SDF, a similar result to what was reported during subgroup analysis for the different SDF testing methods, such as SCD, TUNEL assay and SCSA (21,39,42,43,77-79).
Data on the effect of SDF resulting in a reduced LBR following ICSI is more heterogeneous. Two recent meta-analyses reported non-significant association between sperm DNA damage and LBR after ICSI (3,8). However, this conclusion conflicts with the results of a previous analysis by Osman et al. that reported significantly reduced LBRs with high SDF after ICSI (80). Although mature sperms are able to initiate BER (base excision repair) by 8-oxoguanine DNA glycosylase 1, their subsequent DNA repair mainly depends on oocytes due to lacking the downstream components of this pathway (5,81). Normally, the oocyte with its own DNA repair mechanism can compensate for the damage in sperm DNA. However, if the damage is only partially repaired, embryos formed by the fusion of sperm nuclei with damaged DNA and oocytes may show poor developmental prospects, failing to implant in the uterine lining, or may even be aborted at clinical pregnancy (5,82).
This has also been demonstrated in our meta-analysis, which echoed the above conclusions with an improved CPR, beneficial LBR, and low MR after Testi-ICSI for high level SDF of infertility men. In addition, the decision to adopt testicular sperm for ICSI may require consideration of heterogeneity in the number of cycles, maternal and paternal age, endometrial thickness, ovarian stimulation protocols, the number of retrieved oocytes, the proportion of cycles reaching ET, the number of embryos transferred, and other relevant factors not consistently reported in previous studies that might affect SDF rates and ICSI outcomes. It should be noted that all of the included studies comparing testicular versus ejaculated sperm for ICSI were observational, and the number of studies included in the sub-group analyses was small. Therefore, it is proposed that high-quality studies including different groups of patients are necessary in order to rule out the possibility of confounding variables moderately influencing the strength of these results.
Limitations of this study
As with all meta-analyses, we have to take into consideration some limitations in the present study. Firstly, the number of available studies comparing testicular versus ejaculated sperm for ICSI with high SDF for this study is still limited, and most of the included studies were retrospective (only studies 4, 7, 8 and 9 were prospective). Furthermore, the study population varied, and some studies had low numbers of patients. Interpretation of the evidence from these retrospective studies requires caution because of the possibility of confounding variables influencing the results. Secondly, we also noted that heterogeneity in both the male and female partner with multiple known and unknown factors which might moderately affect the strength of findings regarding ICSI outcomes, were not consistently reported in most of the studies included in our meta-analysis, such as maternal and paternal age, infertility duration, ovarian stimulation protocols, number of oocytes retrieved, use of medication, lifestyle exposures, presence of varicoceles and medication use, along with other relevant male factors. In addition, another important limitation of our study potentially increasing study heterogeneity was the different detection methods and cutoff values that were used to assess SDF, which varied among these studies. Finally, some studies included in our meta-analysis were rated as having moderate or serious risk of bias, largely because of potential confounding bias without adjustment. Furthermore, the low or very low quality rating of the evidence of study outcomes based on the GRADE framework was largely attributed to the nature of the retrospective study design and potential confounding bias. Admittedly, one important limitation of this study was that the current study had not been registered and small biases may exist.
Despite the abovementioned limitations, statistical heterogeneity in ICSI outcomes was low overall, the symmetric funnel plots suggested no significant publication bias, and the subgroup by SDF testing method and sensitivity analyses (e.g., removal of individual studies) revealed minimal differences and indicated a sustained tendency for the direction of effects estimated in our meta-analysis. Additional higher-quality studies, particularly RCTs, are required to help confirm whether Testi-ICSI is truly better for men with high SDF in ejaculated sperm.
Conclusions
Despite the overall low to moderate quality of the studies included, this updated meta-analysis suggests that SDF rates are lower in testicular sperm than in ejaculated sperm and that Testi-ICSI is correlated with better clinical outcomes, including higher CPRs, higher LBRs, and lower MRs, albeit only in selected infertile males with confirmed high post-testicular SDF levels in their ejaculated sperm. It will be useful to assess the implications of DNA damage prior to conducting ICSI in clinical recommendations. However, we encourage further research to standardize methodologies and cut-off values for SDF, and we advise seeking further confirmatory evidence through prospective approaches to assess the influence of DNA fragmentation on ICSI outcomes. Moreover, considering both cost-effectiveness and the potential risks associated with sperm retrieval, treatment with Testi-ICSI should be recommended for use in groups of men with high SDF only after several other strategies to correct underlying factors to alleviate SDF have failed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-415/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-415/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-415/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palermo G, Joris H, Devroey P, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17-8. [Crossref] [PubMed]
- De Geyter C, Calhaz-Jorge C, Kupka MS, et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoz038. [Crossref] [PubMed]
- Ribas-Maynou J, Yeste M, Becerra-Tomás N, et al. Clinical implications of sperm DNA damage in IVF and ICSI: updated systematic review and meta-analysis. Biol Rev Camb Philos Soc 2021;96:1284-300. [Crossref] [PubMed]
- Agarwal A, Barbăroșie C, Ambar R, et al. The Impact of Single- and Double-Strand DNA Breaks in Human Spermatozoa on Assisted Reproduction. Int J Mol Sci 2020;21:3882. [Crossref] [PubMed]
- Ribas-Maynou J, Benet J. Single and Double Strand Sperm DNA Damage: Different Reproductive Effects on Male Fertility. Genes (Basel) 2019;10:105. [Crossref] [PubMed]
- Esteves SC, Roque M, Bradley CK, et al. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril 2017;108:456-467.e1. [Crossref] [PubMed]
- Ambar RF, Agarwal A, Majzoub A, et al. The Use of Testicular Sperm for Intracytoplasmic Sperm Injection in Patients with High Sperm DNA Damage: A Systematic Review. World J Mens Health 2021;39:391-8. [Crossref] [PubMed]
- Deng C, Li T, Xie Y, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: A systematic review and meta-analysis combined with a retrospective cohort study. Andrologia 2019;51:e13263. [Crossref] [PubMed]
- Luo Y, Wu S, Zhang M, et al. Sperm DNA integrity is critically impacted by male age but does not influence outcomes of artificial insemination by husband in the Chinese infertile couples. Aging (Albany NY) 2022;14:4326-35. [Crossref] [PubMed]
- Colpi GM, Francavilla S, Haidl G, et al. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018;6:513-24. [Crossref] [PubMed]
- Schlegel PN, Sigman M, Collura B, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril 2021;115:54-61. [Crossref] [PubMed]
- Zhang Z, Zhu L, Jiang H, et al. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. J Assist Reprod Genet 2015;32:17-26. [Crossref] [PubMed]
- Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev 2017;6:243. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Alharbi M, Hamouche F, Phillips S, et al. Use of testicular sperm in couples with SCSA-defined high sperm DNA fragmentation and failed intracytoplasmic sperm injection using ejaculated sperm. Asian J Androl 2020;22:348-53. [Crossref] [PubMed]
- Zhang J, Xue H, Qiu F, et al. Testicular spermatozoon is superior to ejaculated spermatozoon for intracytoplasmic sperm injection to achieve pregnancy in infertile males with high sperm DNA damage. Andrologia 2019;51:e13175. [Crossref] [PubMed]
- Herrero MB, Lusignan MF, Son WY, et al. ICSI outcomes using testicular spermatozoa in non-azoospermic couples with recurrent ICSI failure and no previous live births. Andrology 2019;7:281-7. [Crossref] [PubMed]
- Arafa M, AlMalki A, AlBadr M, et al. ICSI outcome in patients with high DNA fragmentation: Testicular versus ejaculated spermatozoa. Andrologia 2018;50: [Crossref] [PubMed]
- Pabuccu EG, Caglar GS, Tangal S, et al. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia 2017;49: [Crossref] [PubMed]
- Bradley CK, McArthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Moskovtsev SI, Alladin N, Lo KC, et al. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med 2012;58:142-8. [Crossref] [PubMed]
- Moskovtsev SI, Jarvi K, Mullen JB, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril 2010;93:1142-6. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Lewis SE, O’Connell M, Stevenson M, et al. An algorithm to predict pregnancy in assisted reproduction. Hum Reprod 2004;19:1385-94. [Crossref] [PubMed]
- Bibi R, Jahan S, Afsar T, et al. The influence of paternal overweight on sperm chromatin integrity, fertilization rate and pregnancy outcome among males attending fertility clinic for IVF/ICSI treatment. BMC Pregnancy Childbirth 2022;22:620. [Crossref] [PubMed]
- Amor H, Hammadeh ME, Mohd I, et al. Impact of heavy alcohol consumption and cigarette smoking on sperm DNA integrity. Andrologia 2022;54:e14434. [Crossref] [PubMed]
- Finelli R, Darbandi S, Pushparaj PN, et al. In Silico Sperm Proteome Analysis to Investigate DNA Repair Mechanisms in Varicocele Patients. Front Endocrinol (Lausanne) 2021;12:757592. [Crossref] [PubMed]
- Henkel R, Offor U, Fisher D. The role of infections and leukocytes in male infertility. Andrologia 2021;53:e13743. [Crossref] [PubMed]
- Talibova G, Bilmez Y, Ozturk S. DNA double-strand break repair in male germ cells during spermatogenesis and its association with male infertility development. DNA Repair (Amst) 2022;118:103386. [Crossref] [PubMed]
- Agarwal A, Farkouh A, Parekh N, et al. Sperm DNA Fragmentation: A Critical Assessment of Clinical Practice Guidelines. World J Mens Health 2022;40:30-7. [Crossref] [PubMed]
- Qiu D, Shi Q, Pan L. Efficacy of varicocelectomy for sperm DNA integrity improvement: A meta-analysis. Andrologia 2021;53:e13885. [Crossref] [PubMed]
- Sørensen F, Melsen LM, Fedder J, et al. The Influence of Male Ejaculatory Abstinence Time on Pregnancy Rate, Live Birth Rate and DNA Fragmentation: A Systematic Review. J Clin Med 2023;12:2219. [Crossref] [PubMed]
- Tvrdá E, Ďuračka M, Benko F, et al. Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men-How Does the Seminal Bacteriome Respond?. Int J Mol Sci 2023;24:3503. [Crossref] [PubMed]
- Farkouh A, Salvio G, Kuroda S, et al. Sperm DNA integrity and male infertility: a narrative review and guide for the reproductive physicians. Transl Androl Urol 2022;11:1023-44. [Crossref] [PubMed]
- Sánchez-Martín P, Sánchez-Martín F, González-Martínez M, et al. Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med 2013;59:256-60. [Crossref] [PubMed]
- Marinaro JA, Schlegel PN. Sperm DNA Damage and Its Relevance in Fertility Treatment: A Review of Recent Literature and Current Practice Guidelines. Int J Mol Sci 2023;24:1446. [Crossref] [PubMed]
- Xie P, Keating D, Parrella A, et al. Sperm Genomic Integrity by TUNEL Varies throughout the Male Genital Tract. J Urol 2020;203:802-8. [Crossref] [PubMed]
- Aitken RJ, Drevet JR, Moazamian A, et al. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants (Basel) 2022;11:306. [Crossref] [PubMed]
- Mehta A, Bolyakov A, Schlegel PN, et al. Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil Steril 2015;104:1382-7. [Crossref] [PubMed]
- Mehta A, Esteves SC, Schlegel PN, et al. Use of testicular sperm in nonazoospermic males. Fertil Steril 2018;109:981-7. [Crossref] [PubMed]
- Cheung S, Schlegel PN, Rosenwaks Z, et al. Revisiting aneuploidy profile of surgically retrieved spermatozoa by whole exome sequencing molecular karyotype. PLoS One 2019;14:e0210079. [Crossref] [PubMed]
- Abhyankar N, Kathrins M, Niederberger C. Use of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with cryptozoospermia: a meta-analysis. Fertil Steril 2016;105:1469-1475.e1. [Crossref] [PubMed]
- Caliskan Z, Kucukgergin C, Aktan G, et al. Evaluation of sperm DNA fragmentation in male infertility. Andrologia 2022;54:e14587. [Crossref] [PubMed]
- Zhu C, Zhang S, Chen F, et al. Correlations between elevated basal sperm DNA fragmentation and the clinical outcomes in women undergoing IUI. Front Endocrinol (Lausanne) 2022;13:987812. [Crossref] [PubMed]
- Gill K, Machalowski T, Harasny P, et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants (Basel) 2022;11:1987. [Crossref] [PubMed]
- Alvarez JG, García-Peiró A, Barros A, et al. Double strand DNA breaks in sperm: the bad guy in the crowd. J Assist Reprod Genet 2023;40:745-51. [Crossref] [PubMed]
- Boitrelle F, Shah R, Saleh R, et al. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life (Basel) 2021;11:1368.
- Agarwal A, Majzoub A, Baskaran S, et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J Mens Health 2020;38:412-71. [Crossref] [PubMed]
- Ribas-Maynou J, García-Peiró A, Fernández-Encinas A, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013;1:715-22. [Crossref] [PubMed]
- Simon L, Liu L, Murphy K, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod 2014;29:904-17. [Crossref] [PubMed]
- Esteves SC, Zini A, Coward RM, et al. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia 2021;53:e13874. [Crossref] [PubMed]
- Santi D, Spaggiari G, Simoni M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management – meta-analyses. Reprod Biomed Online 2018;37:315-26. [Crossref] [PubMed]
- Zhang Z, Zhu LL, Jiang HS, et al. Predictors of pregnancy outcome for infertile couples attending IVF and ICSI programmes. Andrologia 2016;48:874-81. [Crossref] [PubMed]
- Ribeiro S, Sharma R, Gupta S, et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017;5:477-85. [Crossref] [PubMed]
- Baskaran S, Agarwal A, Panner Selvam MK, et al. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: a scientometric analysis. Reprod Biol Endocrinol 2019;17:110. [Crossref] [PubMed]
- Belloc S, Benkhalifa M, Cohen-Bacrie M, et al. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril 2014;101:1588-93. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Gosálvez J, Migueles B, López-Fernández C, et al. Single sperm selection and DNA fragmentation analysis: The case of MSOME/IMSI. Natural Science 2013;5:7-14. [Crossref]
- Casanovas A, Ribas-Maynou J, Lara-Cerrillo S, et al. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil Steril 2019;111:699-707.e1. [Crossref] [PubMed]
- Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One 2012;7:e44679. [Crossref] [PubMed]
- Simon L, Murphy K, Shamsi MB, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod 2014;29:2402-12. [Crossref] [PubMed]
- Garolla A, Cosci I, Bertoldo A, et al. DNA double strand breaks in human spermatozoa can be predictive for assisted reproductive outcome. Reprod Biomed Online 2015;31:100-7. [Crossref] [PubMed]
- Simon L, Proutski I, Stevenson M, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online 2013;26:68-78. [Crossref] [PubMed]
- Esbert M, Pacheco A, Vidal F, et al. Impact of sperm DNA fragmentation on the outcome of IVF with own or donated oocytes. Reprod Biomed Online 2011;23:704-10. [Crossref] [PubMed]
- Anifandis G, Bounartzi T, Messini CI, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2015;47:295-302. [Crossref] [PubMed]
- Çağlayan A, Horsanali MO, Buyrukcu BA. The role of sperm DNA integrity in couples with recurrent implantation failure following IVF treatment. Andrologia 2022;54:e14496. [Crossref] [PubMed]
- Zhang H, Zhu FY, He XJ, et al. The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology. Open Life Sci 2023;18:20220597. [Crossref] [PubMed]
- Zhu C, Chen F, Zhang S, et al. Influence of sperm DNA fragmentation on the clinical outcome of in vitro fertilization-embryo transfer (IVF-ET). Front Endocrinol (Lausanne) 2022;13:945242. [Crossref] [PubMed]
- Benchaib M, Braun V, Lornage J, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003;18:1023-8. [Crossref] [PubMed]
- Cozzubbo T, Neri QV, Goldstein M, et al. Topographic mapping of sperm DNA fragmentation within the male genital tract. Fertil Steril 2014;102:e188. [Crossref]
- Liu K, Mao X, Pan F, et al. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci Rep 2023;13:2717. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang Lee R, Li SH, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Gandini L, Lombardo F, Paoli D, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod 2004;19:1409-17. [Crossref] [PubMed]
- Middelkamp S, van Tol HTA, Spierings DCJ, et al. Sperm DNA damage causes genomic instability in early embryonic development. Sci Adv 2020;6:eaaz7602. [Crossref] [PubMed]
- Khadem N, Poorhoseyni A, Jalali M, et al. Sperm DNA fragmentation in couples with unexplained recurrent spontaneous abortions. Andrologia 2014;46:126-30. [Crossref] [PubMed]
- Tan J, Taskin O, Albert A, et al. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online 2019;38:951-60. [Crossref] [PubMed]
- Bareh GM, Jacoby E, Binkley P, et al. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: a prospective study. Fertil Steril 2016;105:329-36.e1. [Crossref] [PubMed]
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Marchetti F, Bishop J, Gingerich J, et al. Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair. Sci Rep 2015;5:7689. [Crossref] [PubMed]
- González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci 2012;13:14026-52. [Crossref] [PubMed]



