Stem cell manipulation, gene therapy and the risk of cancer stem cell emergence
Introduction
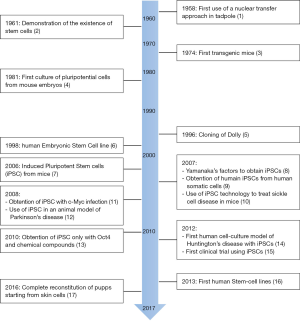
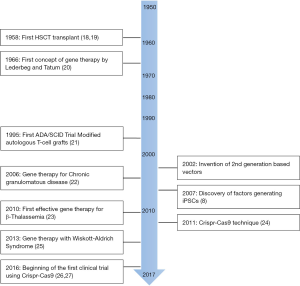
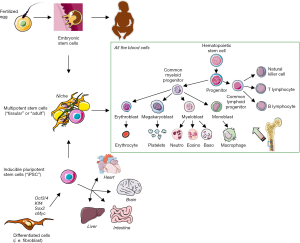
The last few decades have witnessed major achievements in stem cell (SC) manipulation (Figure 1). This is especially true for hematopoietic stem cells (HSCs) due to the development of SC transplantation several decades ago, and more recently to that of gene therapy (GT) (Figure 2). Lately, SC researchers have made a tremendous breakthrough by artificially inducing cell reprogramming, thus increasing the probability of curing genetic diseases using GT. However, despite these new attractive concepts and exciting results, artificial modification of genes is also likely to generate unwanted consequences and requires caution. Therefore, safety procedures remain a fundamental issue in the field. To illustrate these progresses and remaining issues, we will present key examples of the use of HSCs and of GT. We will discuss the “duality” of using mesenchymal stem cells (MSCs) and provide perspectives on novel opportunities brought about by a new era of fetal, pluripotent and mature SCs. We will present the development of associated therapies, including some aspects linked to the arrival of the highly promising CRISPR-Cas9 technology.
HSCs: an outstanding model for regenerative medicine
HSCs: a pioneer tool for regenerative medicine
HSCs are a heterogeneous group of highly plastic adult stem cells (ASCs), which can self-renew and give rise to blood cell lineages. For example, in mice, a single HSC can give rise to epithelial cells of different tissues (28). Similar characteristics of HSCs are maintained in humans (29-31). HSC transplantation (HSCT) has been used since 1958 (32), rendering HSCs the first ASCs successfully used for regenerative medicine to treat leukemic patients. However, as HSCs are very rare, scientists have attempted for years to multiply them without inducing differentiation or to generate them from differentiated cell types, including fibroblasts (33-35). All of these strategies have so far failed to reproduce blood HSC features. However, in the last decade the induced transient expression of six key transcription factors (RUN1T1, HLF, LMO2, PRDM5, PBX1 and ZFP37) finally succeed in producing functional multi-lineages HSCs, as confirmed by their transplantation potential to restore mouse-differentiated blood cells (36). These cells, called induced-HSCs (iHSCs), display significant self-renewal and differentiation potentials at the clonal level. However, expanding the use of iHSCs ex vivo while maintaining their stemness remains very challenging (37). Furthermore, several processes are explored to improve HSC engraftment, including selectin modifications or fucosylation of HSCs (38). Lastly, molecular mechanisms controlling HSC fate determination must be fully deciphered as well as understanding the complexity of HSCs due to their heterogeneity.
GT: a brief insight into a bright future
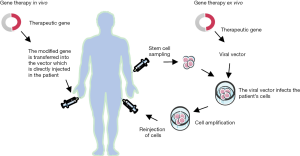
GT consists in transferring in vivo or ex vivo genetic material into cells through a vector to modify transcriptional expression and correct pathological defects (Figure 3). Ex vivo GT trials have only been performed using inactivated viral vectors with an impaired replication. First hematopoietic cell-based gene therapies were performed to treat primary immunodeficiencies, like X-linked severe combined immunodeficiency (SCID) (39) and other genetic disorders (23). These trials achieved mild success as some patients developed leukemia following tumorigenic insertion due to the retrovirus. This was shown to occur frequently at the LMO2 gene promoter site (40). LMO1 and 2 belong to the first proto-oncogenes observed in acute lymphoblastic leukemia-type T (ALL-T) (41,42). This transcription factor is of major importance in primary and definitive hematopoiesis during embryonic stages and was therefore also revealed as implicated in ALL. LMO2 translocation is mediated by V(D)J recombinases RAG1 and RAG2 (43). Mouse models expressing the LMO2 oncogene highlighted its importance to cooperate with another transcription factor called Scl/tal1 in the induction of ALL-T. In the case of GT some patients develop an ALL-T, owing to the integration of the vector near LMO2. It was thus speculated that the integration of the vector near LMO2 was instrumental in initiating the oncogenic process (44).
To reduce the risk of developing leukemogenesis, second generation vectors were created, called “self-inactivating” (SIN) vectors, by inducing a reduction in the adverse transactivation of gene expression. These SIN vectors were then used in a new trial on SCID patients, who displayed no symptoms of leukemia after 4 years (45). If safety is confirmed, GT for SCID patients will become an efficient alternative to haploidentical HSC engraftment (46). New classes of integrating GT vectors are now being developed based on lentiviruses that incorporate SIN safety features to avoid tumorigenic insertion (47).
Gene editing, developed in the last few years, alters DNA sequences using artificially modified nucleases which act as molecular scissors. This process is based on DNA repair mechanisms, namely homology directed repair (HDR) or non-homologous end-joining (NHEJ). Genome editing is limited as high-fidelity HDR only occurs during the G2/S phase, whereas NHEJ induces insertions and deletions. This technique has been used to insert a whole transgene into a defined locus (“safe harbor”) to recover a gene function (45,48). Some studies on HSC-modified zinc finger nucleases (ZFN) were performed to recover the functional expression of IL2RG (mutated in SCID patients) through gene insertion into a safe harbor or downstream of the promoter (49,50). Primitive hematopoietic cells are more sensitive than progenitors to the cytotoxicity of gene targeting procedures and less proficient at performing HDR, because of their quiescence (51). Therefore, in this study, HSC expansion was induced to favor gene editing by HDR. However, despite the high specificity for the IL2R locus, further studies are still required to ensure the absence of off-target changes generated by NHEJ. Determining how to improve HSC transduction rate remains a challenge. Indeed, retroviruses preferentially enter proliferative cells, limiting the number of modified HSCs available for patient engraftment. Importantly, cytokine stimulation can impair bone marrow (BM)-homing and engraftment of CD34+ cells (52). Therefore, a high viral exposure combined to cytokine stimulation could promote mutagenesis and multicopy integration (44). As an alternative strategy, the recent use of a lentiviral vector pseudo-typed with a baboon retroviral envelope glycoprotein resulted in a higher transduction efficiency in quiescent CD34+, at a low concentration without any cytokine stimulation (53).
Altogether, the safety of these different approaches remains questionable. Indeed, NHEJ-mediated gene editing may trigger unwanted modifications. Other techniques are being developed such as genomic insulators that consist of genetic sequences designed to reduce inappropriate gene activation by blocking the ability of enhancers to activate promotors. This seems very promising to reduce insertion mutagenesis. Moreover, a strategy that relies on the insertion of cell type-specific promoters allows a transgene expression restricted to lineage-committed cells. This could be a better strategy to reduce risks of cell transformation when a unique cell lineage is altered, such as in β-thalassemia.
Recent advances in genome editing have been made using a technology derived from the microbial defense system called CRISPR-Cas9. In bacteria, CRISPR-Cas systems provide immunity by incorporating fragments of invading phage and plasmid DNA into CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) loci and using the corresponding CRISPR RNAs to guide the degradation of homologous sequences (54). Cas9 is an endonuclease, associated with CRISPR sequences, able to detect and cleave DNA. It acts as a molecular scissor, for “genome surgery”, enabling the insertion of a sequence of interest. This technology can be applied to correcting genetic mutations, and will certainly become valuable in diverse domains such as understanding the role of genes in biological processes, drug development and pre-clinical use for genome surgery in patients. This apparent “easy-to-use” technology is currently being tested in a phase I clinical trial since 2016 (27). The ease of infecting hematopoietic cells explains why this therapy is initially proposed in monogenic diseases of this tissue. Ongoing GT clinical trials mostly involve cancers then vascular anomalies, and monogenic diseases (55).
Promising potential of MSCs
MSCs definition and properties
MSCs are non-hematopoietic cells of the microenvironment. MSCs are present in most tissues and can be isolated from a variety of different hematopoietic tissues such as bone marrow, as well as from non-hematopoietic adult tissues. The International Society of Cellular Therapy has described the minimum criteria necessary for defining and characterizing multipotent human MSCs in vitro (56,57), such as plastic adherence, morphology, phenotype and potential capacities. Morphologically, MSCs are a heterogeneous population containing cells with a morphology ranging from fibroblast-like to cuboidal (56) and have colony-forming unit-fibroblast (CFU-F) content. Phenotypically, no specific surface antigen marker combination has been defined for MSC populations. However, MSCs express neither markers of hematopoietic lineages (CD34, CD45, glycophorin A, CD11a, CD14, HLA-DR) nor markers of endothelial lineages (CD11b, CD31), though they express CD29, CD44, CD49, CD51, CD62, CD73, CD90, CD105, CD117, CD166, CD271 and Stro-1 antigens (56). At the functional level, the fact that MSCs can differentiate into bone, cartilage and fat under appropriate stimulatory conditions, represents the major critical requirement to identify putative MSCs population in vitro. Moreover, under certain culture conditions, they can differentiate into dopaminergic neurons, pancreas, cardiac and lung cells, astrocytes and endothelial cells (56,58-60).
An attempt to define the MSC compartment unveiled an initial controversial matter, since cells matching the above-mentioned criteria do not represent a unique cell population but rather a combination of heterogeneous cell types (56,61-63).
Distinct studies revealed the remarkable property of MSCs to spontaneously home to injured sites where they actively participate in tissue regeneration. Interestingly, MSCs have therapeutic benefits due to their ability to act as a trophic factor by delivering many growth factors or bioreactive factors. These include antioxidants, pro-angiogenic substances, and cytokines that induce DNA repair by limiting apoptosis and stress responses by mobilizing reparative functions and recruiting immune cells of the recipient (64). These properties render MSCs highly interesting for the treatment of many diseases. MSCs can secrete multiple paracrine growth factors/cytokines involved in inflammation and modulate adaptive immune cells at different levels of the immune response, including in the reprogramming of monocytes/macrophages, in the interference with dendritic cell differentiation, maturation, and function, in modulating natural killer cells, and in T cell activation and suppression of proliferation (64-66). MSCs can support neo-angiogenesis to promote re-vascularization of regenerated tissue (67,68). Indeed, they have been shown to directly promote neo-vessel formation (69,70). Finally, MSCs can support tissue-specific SCs differentiation such as hematopoiesis (71), and have been reported to support HSC maintenance and engraftment (72).
MSCs in clinical applications
MSC-based therapies have been shown to be efficient in preclinical studies in tissue engineering and regenerative medicine for the treatment of several pathologies including cartilage, skin wounding, bone injuries, liver failure (ALF), myocardial infarction, nervous diseases, kidney (AKI) and pulmonary fibrosis (ARDS). To date, there are more than 500 MSC-related clinical research protocols listed in www.clinicaltrials.gov that represent over 660 different conditions and more than 2,000 MSC patients treated worldwide.
In addition, MSCs could also be used as a delivery platform for therapeutic agents (73). Most of the clinical trials are now in phase I/II and so far, appear to be safe. For example, MSC therapies were recently used in the context of a retinal and optic nerve disease (74), for chronic lung allograft dysfunction (75). It was also used for the regeneration of durable articular cartilage in osteoarthritic knees where no cases of osteogenesis or tumorigenesis were observed after 7 years (76). Moreover, MSCs have been used as therapy for chronic obstructive pulmonary disease (COPD) (77) and in congestive heart failure cardiopoietic regenerative therapy (CHART-1) (78).
Technical problems associated with the use of MSCs
Prior to their use in therapies, MSCs need to be isolated from various tissues from multiple origins by various purification techniques. This is a key issue to achieve standardization of MSC isolation protocols. In addition, the heterogeneity of MSCs influences the properties of in vitro expanded MSCs. Next, during in vitro expansion, only a limited number of MSC clones are capable of long-term expansion, and unfortunately, they lose their multipotent potential during this process. MSCs are highly exposed to spontaneous transformation during this proliferation phase in culture. Moreover, the choice of the route of delivery (intravenous injection or intra-arterial local injection) and location of MSCs may affect their efficient trafficking and homing to injured organs. So far, it is very difficult to assess the impact of MSC production and MSC sources on clinical outcome as very few comparable studies have been reported. Therefore, it is important to homogenize and standardize procedures to delimit the conditions and parameters used in the different experiments/trials and choose a unique delivery procedure for further therapeutic consideration.
Different studies have demonstrated the role of MSCs in tumorigenesis. In general, it is believed that MSCs affect tumor growth and invasion through different mechanisms such as the expression of growth factors, increased angiogenesis and metastasis, and/or through modifications in the microenvironment (79,80). MSCs can differentiate into different cell types, such as adipocytes, which develop a pro-tumorigenic activity, or into osteoblasts, which are involved in drug resistance since they can protect leukemic cells from chemotherapy-induced apoptosis via increased engraftment of leukemic cells in the BM (81). However, there is also growing evidence that MSCs increase or inhibit the growth and invasion of tumors through direct or indirect interaction with tumor cells (82). Hence, the mechanisms involved in these processes remain unclear/controversial and there is a real need for further comprehensive studies. Concerning the specific role of MSC alterations in the niche during leukemogenesis, there is no direct evidence demonstrating that an initial lesion in MSCs may play a causative role in human leukemia. For instance, an impaired expression of the ribosome maturation protein SBDS, the ribonuclease Dicer, and the endoribonuclease Drosha, was described in MSCs but not in hematopoietic cells from myelodysplastic syndrome (MDS) patients in comparison with healthy donors (83,84).
The role of the niche, and in particular that of MSCs is the focus of a growing number of studies highlighting that there is a real crosstalk between the niche and leukemic cells (85). Medyouf and colleagues have shown that, in comparison with age-matched counterparts, healthy MSCs significantly enhance MDS CD34+ engraftment in vivo (86), likely owing to the factors differentially expressed between MDS and healthy MSCs such as LIF (87,88), VEGFA (89), IGFBP2 (90), and N-Cadherin (86). Moreover, some alterations in niche cells are sufficient to drive the development of myeloid malignancies in mice (91-93). In contrast, leukemic cells can alter their niche counterpart in genetic mouse models of chronic (CML) and acute myelogenous leukemia (AML) (94).
It was also reported that loss of MSCs, with the associated reduction in CXCL12, is sufficient to accelerate myeloproliferative neoplasm (MPN) progression (95). This phenomenon is reversible and demonstrates that niche transformation represents a major driving force and a requirement for disease progression, and provides a novel, potentially safe therapeutic approach, in which hematopoietic cell-directed therapies were previously shown to be of limited efficacy. Hence, different studies have highlighted that the microenvironment is a possible therapeutic target in AML (96-98).
From embryonic stem cells (ESCs) to induced pluripotent SCs
ESCs
ESCs are constitutive pluripotent cells that originate from the inner cell mass of mammalian blastocysts (5–7 days after fertilization), giving rise to the three germinal layers (endoderm, ectoderm, and mesoderm). ESCs grow in tight colonies, using a feeder layer, and remain undifferentiated indefinitely under defined conditions (99). They spontaneously differentiate into so-called embryonic bodies when cultivated in vitro. In vivo, this spontaneous evolution of ESCs toward differentiation is clearly observed when they are implanted into immunosuppressed mice, forming teratomas, in which cells evolve independently along the differentiation process and grow randomly into the three germinal layers (100). After several passages, chromosome abnormalities appear leading to malignancy (101). This tumorigenic potential is present in a wide range of established tumors, and increases with the number of passages. In addition, ESCs display alterations of their karyotype when cultured for extended periods of time, and highly-passaged cells tend to form less mature tumors. Specific genes affected are linked to cancer and are located on chromosomes involved in culture adaptation. The balance between the generation of enough cells which implies an increased risk of teratoma formation (i.e., potential risk of developing cancer in treated patients) and the use of naïve ESCs that form more mature teratomas (less aggressive, i.e., decrease the level of aggressiveness of cancer cells and their potential transformation) need further investigation. However, if the objective is to prevent all risks of transformation, the technique remains limited in terms of the quantity of cells produced. Altogether it creates a subtly balanced situation for the use of human ESCs in regenerative medicine. Interestingly, neither euploid (abnormality-free cells) nor in vitro cultured ESCs develop teratomas after complete differentiation before transplantation. This discovery could be exploited by exclusively removing tumorigenic-prone cells from differentiated ones, based for example on the use of several fluorescent differentiation markers/probe couples.
Clinical trials have been initiated to explore the potential of ESCs as candidates for regenerative medicine. For instance, ESC therapies are currently tested to treat age-related macular degeneration (ARMD) and spinal cord injury (102).
Adult SCs
Other SCs, such as placental, neural or vascular endothelial SCs are of interest in terms of regenerative medicine. Those SCs can be defined as multipotent SCs which give rise to more than one cell type pertaining to a specific cell lineage. Derived from placenta, fetal SCs such as amniotic/chorionic mesenchymal cells and chorionic mesenchymal stromal/trophoblast cells have been shown to differentiate into the three germinal layers [thus including osteogenic, neurogenic, myogenic, adipogenic, pulmonary, cardiac, endothelial, pancreatic or hepatogenic cell types (103)]. To date, placental SC therapeutic applications range from cardiovascular, musculoskeletal, liver, neurological, liver, ocular surface diseases to recent clinical trials investigating their therapeutic use in Crohn’s disease, sclerosis, pulmonary sarcoidosis, hematologic disorders, myelodysplasia, graft versus host disease (103,104). Neural stem cells (NSCs), isolated from the adult sub ventricular zone have recently prompted the interest of regenerative medicine researchers, as they display properties of proliferation, self-renewal and differentiation into different mature cell types (105). Several trials are under progress to investigate the therapeutic use of NSCs in damaged central nervous systems, such as strokes, spinal cord injuries, and degenerative diseases (106). Despite promising results in clinical trials, further research on NSCs remains to be conducted, as adult NSCs represent a highly heterogeneous pool of cells, and as the signaling pathways involved in the regulation of NSC properties are still poorly understood (107). Derived from either bone marrow or peripheral blood autologous sources, vascular endothelial SCs (VESCs) are multipotent SCs currently under study in different clinical trials for their potential therapeutic use in hypertension, refractory angina or limb ischemia, thus highlighting the promising potential of VESCs in treating cardiovascular diseases (106). These adult multipotent SCs may lead to the development of autologous regenerative medicine without raising the ethical issues associated with the use of ESCs.
Induced pluripotent stem cells (iPSCs)
iPSCs are derived from differentiated somatic cells such as fibroblasts that are reprogrammed following genetic modifications or chemical treatments, to return to a pluripotent SC stage. In 2006, for the first time, Yamanaka and colleagues successfully reprogrammed fully differentiated cells into iPSCs (7) using 4 genes involved in the maintenance of ESC pluripotency, namely Oct3/4, Sox2, c-Myc, and Klf4. In 2007, this technique was reproduced in rats (108,109), monkeys (110), and human fibroblasts (8). iPSCs can be re-directed towards different desired cell types. Importantly, iPSCs can form teratomas and like ESCs can contribute to all cell lineages when injected into mouse blastocysts, such as cardiac or neural cells (111). The former were obtained by exposing iPSC to activin A and bone morphogenic protein 4 (BMP4) (112). Dopaminergic-like neural cells are obtained by exposing iPSCs to the extrinsic control of microenvironment stromal cell-derived inducing activity (113). Blood and neural immature cells have a predisposition for reprogramming and do so in a more efficient manner, probably due to their epigenetic memory (114-116). The difference between iPSCs and ESCs initially appears to be very slight (Figure 4), especially when considering molecular factors involved in their regulation (99). However, several biological features differ. One of the most remarkable differences in the context of cell therapy resides in the fact that long culture periods do not create detectable alterations in iPSCs.
Several attempts have been made to transfer the reprogramming technology to clinical applications but have been interrupted due to non-clinical issues. In 2014, a Japanese team of surgeons and ophthalmologists successfully implanted cells derived from iPSC on a 70-year-old woman who suffered from an ARMD. A de novo retinal epithelium of only 1.3-by-3 mm2 was successfully implanted (117). Although this procedure did not attempt to restore the vision of the patient, since this is very unlikely, it nevertheless provided an opportunity to monitor side effects, immunogenicity or cancer growth in a human patient. To date it constitutes the first clinical trial on a practical aspect of the iPSCs with a positive outcome, illustrating current hopes in regenerative therapies as evidenced by several other ongoing clinical trials for ARMD treatments using iPSCs or ESCs (118,119). Moreover, new prospects for the treatment of hypertension using iPSCs have recently been described (120). Mechanistically, patient-specific iPSCs currently provide a powerful tool to dissect human single genetic mutation diseases such as familial platelet disorder (121). For example, patient-derived iPSCs are considered to be a new clinical approach to rescue the hemoglobin β gene mutation responsible of β-thalassemia using the CRISPR/Cas9 technology (122).
Unfortunately, frequent teratomas and teratocarcinomas are observed when iPSCs are re-implanted into immunodeficient mice (8). This problem can be partly overcome by introducing Nanog-iPSC, in which the four factors (Klf4, Oct3/4, cMyc, Sox2) are silenced after de-differentiation, whereas the Nanog expression level is maintained. Data using the silencing of the four factors in Nanog-iPSCs demonstrated that these factors are only involved in the induction of pluripotency, but not in the subsequent maintenance of stemness properties. This observation offers opportunities to develop new designs of cancer-free regenerative medicine protocols, switching from a stable expression system (retroviral-based) to a transient one (adenovirus-based), and thus excluding a teratoma-prone environment due to the expression of c-Myc (7).
Differentiation of iPSCs or ESCs into HSCs is currently considered to be another option to generate and experiment on a large number of human HSCs, but the methodologies used produce multipotent progenitors with only a short-term repopulating potential (123). BM stromal-derived iPSCs allow erythroid cell generation with a phenotype closely resembling that of in vivo cells, thereby providing a powerful tool to study erythropoiesis or replacing red blood cell transfusion (124). Recently, monkey iPSC-derived neutrophils have been generated (125). Current efforts are focusing on the development of novel 3D-culture supports for the large-scale culture of inducible HSCs (iHSCs) (126). However, none of these iHSCs have so far been approved for medical use, and a new trial for blood cell generation from iPSCs should start in 2017 (127).
Conclusions & Discussion
Cancer SCs can either originate from true SC transformation or from de-differentiated mature cells (128,129). It is therefore possible that cancer SCs originate from a spontaneous in vivo-in situ reprogramming of either adult cells or de-differentiated mature cells (129,130). In this context, in order to transform an experimental approach into a feasible clinical application, efforts have recently been made to increase the number of re-programmed cells, either by working on the efficiency of the conversion process, to reach a deterministic process (100% efficiency) (131), or by designing simpler culture conditions, independently of the nature of the somatic cells used. The first option has been explored through chromatin remodeling and DNA acetylation mechanisms. Repression of methyl-CpG binding domain protein 3 (MBD3) expression, a subunit of the Mir-2/NuRD complex (an ATP-dependent chromatin remodeling and histone deacetylase complex), was shown to drastically increase the efficiency of the technique, almost reaching 100% within 7 days. Different teams have been able to induce pluripotent SCs using a cocktail of purified proteins derived from the four genes reported by Yamakana, thus avoiding the use of DNA for reprogramming (108,132).
A promising strategy lies in the use of the CRISPR/Cas9 technology, which allows the correction of a genetic mutation, and is also known as “genome surgery”. Following preliminary in vitro and in vivo results, clinical trials are ongoing (27). Today, GT clinical trials are focusing first on cancers, then on vascular anomalies, and on monogenic diseases (55).
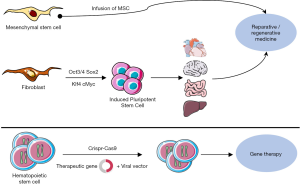
In conclusion, the last decades have provided a wealth of novel basic, conceptual and technical knowledge and major advances in regenerative medicine (Figure 5). This upsurge in cell therapy investigations led to renewed perspectives in patient care, with an incredibly broad range of applications in numerous medical fields (Table 1). However, initial experiments and clinical trials also revealed major issues that remain to be addressed. This is especially true regarding the impact of cell-based therapeutic approaches on the risk of cancer development and SC transformation. Both the scientific and medical communities are now aware of these problems and limitations, and the challenges that lie ahead have now been clearly identified. Current investigations and new approaches based on recent advances in the biological understanding of mechanisms controlling SC fate, including epigenetic mechanisms and vectorology, are improving the tools and strategies, to ultimately influence clinical outcome. Therefore, cell therapy is becoming one of the major therapeutic “weapons” to tackle cancer development and to prolong the human lifespan under acceptable conditions, thus drawing us nearer to achieving our wildest dream.
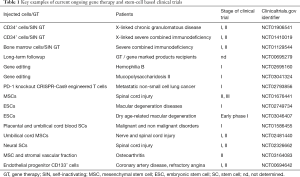
Full table
Acknowledgments
We thank Dr. Brigitte Manship for critical reading and language editing assistance.
Funding: This study was funded by Canceropôle Rhône-Auvergne (CLARA), Région Auvergne-Rhône-Alpes; Fondation de France 2014-0047501; “Déchaîne Ton Coeur”, “La Ligue Nationale Contre le Cancer” (National, Departemental Commitee of Ain, Rhône and Saône-et-Loire), “la Fondation ARC pour la Recherche sur le Cancer” fundation and INCA (SEIN14-010) grants.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus l aevis from the transplantation of single somatic nuclei. Nature 1958;182:64-5. [Crossref] [PubMed]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 1961;14:213-22. [Crossref] [PubMed]
- Jaenisch R, Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A 1974;71:1250-4. [Crossref] [PubMed]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292:154-6. [Crossref] [PubMed]
- Campbell KH, McWhir J, Ritchie WA, et al. Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996;380:64-66. [Crossref] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Yu J, Vodyanik MA, Smug-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917-20. [Crossref] [PubMed]
- Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920-3. [Crossref] [PubMed]
- Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 2008;26:101-6. [Crossref] [PubMed]
- Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A 2008;105:5856-61. [Crossref] [PubMed]
- Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4:381-4. [Crossref] [PubMed]
- HD iPSC Consortium. Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell 2012;11:264-78. [Crossref] [PubMed]
- Ring KL, Tong LM, Balestra ME, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012;11:100-9. [Crossref] [PubMed]
- Tachibana M, Amato P, Sparman M, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013;153:1228-38. [Crossref] [PubMed]
- Hikabe O, Hamazaki N, Nagamastu G, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016;539:299-303. [Crossref] [PubMed]
- Mathe G, Jammet H, Pendic B, et al. Transfusions and grafts of homologous bone marrow in humans after accidental high dosage irradiation. Rev Fr Etud Clin Biol 1959;4:226-38. [PubMed]
- Mathe G, Hartmann L, Loverdo A, et al. Attempt at protection against radiogold-induced mortality by injection of isologous or homologous bone marrow cells. Rev Fr Etud Clin Biol 1958;3:1086-7. [PubMed]
- Campbell TL. Reflections on research and the future of medicine. Science 1966;153:442-9. [Crossref] [PubMed]
- Blaese RM, Culver KW, Miller AD, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 1995;270:475-80. [Crossref] [PubMed]
- Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006;12:401-9. [Crossref] [PubMed]
- Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 2010;467:318-22. [Crossref] [PubMed]
- Deltcheva E, Chylinski K, Sharma CM, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011;471:602-7. [Crossref] [PubMed]
- Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013;341:1233151. [Crossref] [PubMed]
- Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nature 2016;535:476-7. [Crossref] [PubMed]
- Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature 2016;539:479. [Crossref] [PubMed]
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369-77. [Crossref] [PubMed]
- Körbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002;346:738-46. [Crossref] [PubMed]
- Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000;406:257. [Crossref] [PubMed]
- Mezey E, Key S, Vogelsang G, et al. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A 2003;100:1364-9. [Crossref] [PubMed]
- Mathe G, Bernard J. Bull Assoc Fr Etud Cancer 1958;45:289-300. [Trial therapy, by x-irradiation followed by the administration of homologous bone marrow cells, of highly-advanced spontaneous leukemia in AK mice]. [PubMed]
- Szabo E, Rampalli S, Risueno RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 2010;468:521-6. [Crossref] [PubMed]
- Pereira CF, Chang B, Qiu J, et al. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell 2013;13:205-18. [Crossref] [PubMed]
- Doulatov S, Vo LT, Chou SS, et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 2013;13:459-70. [Crossref] [PubMed]
- Riddell J, Gazit R, Garrison BS, et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 2014;157:549-64. [Crossref] [PubMed]
- Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci 2012;1266:138-50. [Crossref] [PubMed]
- Narasipura SD, Wojciechowski JC, Charles N, et al. P-Selectin coated microtube for enrichment of CD34+ hematopoietic stem and progenitor cells from human bone marrow. Clin Chem 2008;54:77-85. [Crossref] [PubMed]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000;288:669-72. [Crossref] [PubMed]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003;302:415-9. [Crossref] [PubMed]
- Royer-Pokora B, Loos U, Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene 1991;6:1887-93. [PubMed]
- Boehm T, Foroni L, Kaneko Y, et al. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A 1991;88:4367-71. [Crossref] [PubMed]
- Garcia IS, Kaneko Y, Gonzalez-Sarmiento R, et al. A study of chromosome 11p13 translocations involving TCR beta and TCR delta in human T cell leukaemia. Oncogene 1991;6:577-82. [PubMed]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 2008;118:3132-42. [Crossref] [PubMed]
- Hacein-Bey-Abina S, Pai S-Y, Gaspar HB, et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 2014;371:1407-17. [Crossref] [PubMed]
- Touzot F, Moshous D, Creidy R, et al. Faster T-cell development following gene therapy compared with haploidentical HSCT in the treatment of SCID-X1. Blood 2015;125:3563-9. [Crossref] [PubMed]
- Biffi A, Bartolomae CC, Cesana D, et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood 2011;117:5332-9. [Crossref] [PubMed]
- Moehle EA, Rock JM, Lee YL, et al. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci U S A 2007;104:3055-60. [Crossref] [PubMed]
- Lombardo A, Genovese P, Beausejour CM, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 2007;25:1298-306. [Crossref] [PubMed]
- Urnov FD, Miller JC, Lee YL, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 2005;435:646-51. [Crossref] [PubMed]
- Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 2014;510:235-40. [Crossref] [PubMed]
- Ahmed F, Ings SJ, Pizzey AR, et al. Impaired bone marrow homing of cytokine-activated CD34+ cells in the NOD/SCID model. Blood 2004;103:2079-87. [Crossref] [PubMed]
- Girard-Gagnepain A, Amirache F, Costa C, et al. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood 2014;124:1221-31. [Crossref] [PubMed]
- Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods 2013;10:957-63. [Crossref] [PubMed]
- Naldini L. Gene therapy returns to centre stage. Nature 2015;526:351-60. [Crossref] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [Crossref] [PubMed]
- Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393-5. [Crossref] [PubMed]
- Koh SH, Kim KS, Choi MR, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res 2008;1229:233-48. [Crossref] [PubMed]
- Pijnappels DA, Schalij MJ, Ramkisoensing AA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res 2008;103:167-76. [Crossref] [PubMed]
- Lee JW, Gupta N, Serikov V, et al. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther 2009;9:1259-70. [Crossref] [PubMed]
- Han ZC, Du WJ, Han ZB, et al. New insights into the heterogeneity and functional diversity of human mesenchymal stem cells. Biomed Mater Eng 2017;28:S29-S45. [Crossref] [PubMed]
- Horwitz EM, Keating A. Nonhematopoietic mesenchymal stem cells: what are they? Cytotherapy 2000;2:387-8. [Crossref] [PubMed]
- Keating A. Mesenchymal stromal cells. Curr Opin Hematol 2006;13:419-25. [Crossref] [PubMed]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 2009;17:939-46. [Crossref] [PubMed]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84. [Crossref] [PubMed]
- Monsel A, Zhu Y-G, Gennai S, et al. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014;121:1099-121. [Crossref] [PubMed]
- Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005;332:370-9. [Crossref] [PubMed]
- Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 2008;111:4551-8. [Crossref] [PubMed]
- Xu W, Zhang X, Qian H, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623-31. [Crossref] [PubMed]
- Pacini S, Petrini I. Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front Cell Dev Biol 2014;2:20. [Crossref] [PubMed]
- Ringdén O, Remberger M, Svahn BM, et al. Allogeneic hematopoietic stem cell transplantation for inherited disorders: experience in a single center. Transplantation 2006;81:718-25. [Crossref] [PubMed]
- Noort WA, Kruisselbrink AB. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 2002;30:870-8. [Crossref] [PubMed]
- Hurwitz DR, Kirchgesser M, Merrill W, et al. Systemic delivery of human growth hormone or human factor IX in dogs by reintroduced genetically modified autologous bone marrow stromal cells. Hum Gene Ther 1997;8:137-56. [Crossref] [PubMed]
- Labrador-Velandia S, Alonso-Alonso ML, Alvarez-Sanchez S, et al. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J Stem Cells 2016;8:376-83. [Crossref] [PubMed]
- Chambers DC, Enever D, Lawrence S, et al. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl Med 2017;6:1152-7. [Crossref] [PubMed]
- Park YB, Ha CW, Lee CH, et al. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med 2017;6:613-21. [Crossref] [PubMed]
- de Oliveira HG, Cruz FF, Antunes MA, et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl Med 2017;6:962-9. [Crossref] [PubMed]
- Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 2017;38:648-60. [PubMed]
- Ramdasi S, Sarang S, Viswanathan C. Potential of Mesenchymal Stem Cell based application in Cancer. Int J Hematol Oncol Stem Cell Res 2015;9:95-103. [PubMed]
- Arango-Rodriguez ML, Ezquer F, Ezquer M, et al. Could cancer and infection be adverse effects of mesenchymal stromal cell therapy? World J Stem Cells 2015;7:408-17. [Crossref] [PubMed]
- Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007;25:1315-21. [Crossref] [PubMed]
- Norozi F, Ahmadzadeh A, Shahrabi S, et al. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol 2016;37:11679-89. [Crossref] [PubMed]
- Santamaría C, Muntión S, Rosón B, et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica 2012;97:1218-24. [Crossref] [PubMed]
- Sánchez-Aguilera A, Méndez-Ferrer S. The hematopoietic stem-cell niche in health and leukemia. Cell Mol Life Sci 2017;74:579-90. [Crossref] [PubMed]
- Korn C, Mendez-Ferrer S. Myeloid malignancies and the microenvironment. Blood 2017;129:811-22. [Crossref] [PubMed]
- Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell 2014;14:824-37. [Crossref] [PubMed]
- da Silva CL, Goncalves R, Crapnell KB, et al. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol 2005;33:828-35. [Crossref] [PubMed]
- Escary JL, Perreau J, Dumenil D, et al. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature 1993;363:361-4. [Crossref] [PubMed]
- Rehn M, Olsson A, Reckzeh K, et al. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood 2011;118:1534-43. [Crossref] [PubMed]
- Huynh H, Zheng J, Umikawa M, et al. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood 2011;118:3236-43. [Crossref] [PubMed]
- Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 2014;506:240-4. [Crossref] [PubMed]
- Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010;464:852-7. [Crossref] [PubMed]
- Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007;129:1097-110. [Crossref] [PubMed]
- Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013;13:285-99. [Crossref] [PubMed]
- Arranz L, Sanchez-Aguilera A, Martin-Perez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014;512:78-81. [PubMed]
- Zeng Z, Shi YX, Tsao T, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood 2012;120:2679-89. [Crossref] [PubMed]
- Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012;119:3917-24. [Crossref] [PubMed]
- Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 2009;113:6206-14. [Crossref] [PubMed]
- Narsinh KH, Plews J, Wu JC. Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther 2011;19:635-8. [Crossref] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Baker DE, Harrison NJ, Maltby E, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol 2007;25:207-15. [Crossref] [PubMed]
- Aboody K, Capela A, Niazi N, et al. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron 2011;70:597-613. [Crossref] [PubMed]
- Antoniadou E, David AL. Placental stem cells. Best Pract Res Clin Obstet Gynaecol 2016;31:13-29. [Crossref] [PubMed]
- Farmer D. Placental stem cells: The promise of curing diseases before birth. Placenta 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Gil-Perotín S, Duran-Moreno M, Cebrián-Silla A, et al. Adult neural stem cells from the subventricular zone: a review of the neurosphere assay. Anat Rec (Hoboken) 2013;296:1435-52. [Crossref] [PubMed]
- Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015;17:11-22. [Crossref] [PubMed]
- Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015;17:385-95. [Crossref] [PubMed]
- Li W, Wei W, Zhu S, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 2009;4:16-9. [Crossref] [PubMed]
- Liao J, Cui C, Chen S, et al. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 2009;4:11-5. [Crossref] [PubMed]
- Liu H, Zhu F, Yong J, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 2008;3:587-90. [Crossref] [PubMed]
- Skalova S, Svadlakova T, Shaikh Qureshi WM, et al. Induced pluripotent stem cells and their use in cardiac and neural regenerative medicine. Int J Mol Sci 2015;16:4043-67. [Crossref] [PubMed]
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015-24. [Crossref] [PubMed]
- Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000;28:31-40. [Crossref] [PubMed]
- Eminli S, Foudi A, Stadtfeld M, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet 2009;41:968-76. [Crossref] [PubMed]
- Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008;454:646-50. [Crossref] [PubMed]
- Meng X, Neises A, Su RJ, et al. Efficient reprogramming of human cord blood CD34+ cells into induced pluripotent stem cells with OCT4 and SOX2 alone. Mol Ther 2012;20:408-16. [Crossref]
- Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature 2014;513:287-8. [Crossref] [PubMed]
- Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol 2015;33:890-1. [Crossref] [PubMed]
- Fields M, Cai H, Gong J, et al. Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD). Cells 2016;5:E44. [Crossref] [PubMed]
- Biel NM, Santostefano KE, DiVita BB, et al. Vascular Smooth Muscle Cells From Hypertensive Patient-Derived Induced Pluripotent Stem Cells to Advance Hypertension Pharmacogenomics. Stem Cells Transl Med 2015;4:1380-90. [Crossref] [PubMed]
- Nakajima H. Application of iPS cells derived from congenital myelodysplastic syndrome for research of nomal hematopoesis and hematological malignancies. Rinsho Ketsueki 2016;57:1087-94. [PubMed]
- Niu X, He W, Song B, et al. Combining Single Strand Oligodeoxynucleotides and CRISPR/Cas9 to Correct Gene Mutations in beta-Thalassemia-induced Pluripotent Stem Cells. J Biol Chem 2016;291:16576-85. [Crossref] [PubMed]
- Hole N, Graham GJ, Menzel U, et al. A limited temporal window for the derivation of multilineage repopulating hematopoietic progenitors during embryonal stem cell differentiation in vitro. Blood 1996;88:1266-76. [PubMed]
- Uchida N, Haro-Mora JJ, Fujita A, et al. Efficient Generation of β-Globin-Expressing Erythroid Cells Using Stromal Cell-Derived Induced Pluripotent Stem Cells from Patients with Sickle Cell Disease. Stem Cells 2017;35:586-96. [Crossref] [PubMed]
- Schrimpf C, Wrede C, Glage S, et al. Differentiation of induced pluripotent stem cell-derived neutrophil granulocytes from common marmoset monkey (Callithrix jacchus). Transfusion 2017;57:60-9. [Crossref] [PubMed]
- Sugimine Y, Niwa A, Matsubara H, et al. A portable platform for stepwise hematopoiesis from human pluripotent stem cells within PET-reinforced collagen sponges. Int J Hematol 2016;104:647-60. [Crossref] [PubMed]
- Mittra J, Tait J, Mastroeni M, et al. Identifying viable regulatory and innovation pathways for regenerative medicine: a case study of cultured red blood cells. N Biotechnol 2015;32:180-90. [Crossref] [PubMed]
- Sell S. Cellular origin of cancer: dedifferentiation or stem cell maturation arrest? Environ Health Perspect 1993;101 Suppl 5:15-26. [Crossref] [PubMed]
- Trosko JE. Human adult stem cells as the target cells for the initiation of carcinogenesis and for the generation of "cancer stem cells". Int J Stem Cells 2008;1:8-26. [Crossref] [PubMed]
- Trosko JE. Induction of iPS cells and of cancer stem cells: the stem cell or reprogramming hypothesis of cancer? Anat Rec (Hoboken) 2014;297:161-73. [Crossref] [PubMed]
- Rais Y, Zviran A, Geula S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature 2013;502:65-70. [Crossref] [PubMed]
- Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature 2008;453:338-44. [Crossref] [PubMed]
Cite this article as: Clément F, Grockowiak E, Zylbersztejn F, Fossard G, Gobert S, Maguer-Satta V. Stem cell manipulation, gene therapy and the risk of cancer stem cell emergence. Stem Cell Investig 2017;4:67.