Risk factors for poor outcome in posterior reversible encephalopathy syndrome: systematic review and meta-analysis
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinical and imaging findings syndrome with multiple clinical manifestations, characterized by vasogenic edema classically in the posterior circulation territory. Associated symptoms may completely resolve after prompt symptomatic treatment (1-3). Over 20 years have elapsed since PRES was first described by Hinchey et al. in 1996 (4). As our understanding of this disease has gradually improved, its etiology, clinical and imaging spectrum were more fully and accurately described. The clinical symptoms of PRES typically include encephalopathy, seizures, headache, visual symptoms, and focal neurologic deficits (3,5,6). The risk factors for developing PRES include hypertension, pregnancy and puerperal diseases, organ transplantation, immunosuppressive agents or cytotoxic agents, acute or chronic kidney disease, autoimmune diseases, infections, endocrine diseases, etc. (6-8). When potential triggers are eliminated or blood pressure is controlled, the clinical manifestations and imaging findings of PRES are often reversible (9). Even so, many patients still develop permanent neurologic deficits or may even result in death (10,11).
The role of these various risk factors, imaging findings, and biochemical parameters on the prognosis of PRES remains unclear. Previous studies have correlated the etiologies, location of lesions, atypical imaging findings, and some of the biochemical parameters [lactate dehydrogenase (LDH), hyperlipidemia] with the clinical outcome of PRES, in different confounding settings (12-16). However, the results of these studies are variable and incomplete. Using meta-analysis, we have performed a systematic review of multiple retrospective cohort studies to evaluate the relationship between different etiologies, imaging findings, biochemical parameters and clinical outcomes in patients with PRES.
Methods
Search strategy
The eligible studies in the studies were identified by searching the PubMed, Embase, Cochrane Library, and Web of Science databases update to November 2017. The keywords “posterior reversible encephalopathy syndrome” “PRES” “reversible posterior leukoencephalopathy syndrome” “RPLS” “hypertensive encephalopathy” “hyperperfusion encephalopathy” “occipital-parietal encephalopathy” or “reversible posterior cerebral edema encephalopathy” combined with “prognosis” “outcome” “survival” or “mortality” were used to identify studies that access the associations between risk factors and clinical outcomes in patients with PRES. After proper training, the search was conducted by two individuals independently. For all these documents, titles and abstracts were checked, and the entire documents were checked when necessary. If there were differing opinions between the two reviewers, the arbitration was performed by a third person. The references for the selected publications and related reviews were also checked to identify any studies missed in the literature search. Only studies reported in English were retrieved.
Selection strategy
Inclusion criteria were used to screen the relevant literature. First, all the included studies had to evaluate the correlation between related risk factors (including clinical etiology and symptoms, imaging findings and laboratory parameters) and clinical outcomes in patients with PRES. Second, all included studies were observational cohort studies with the sample size no less than 10. Third, the included studies had to report the odds ratio (OR) and its 95% confidence interval (CI) for related risk factors. Alternatively, the studies had to provide sufficient data that could be used to calculate the OR and its 95% CI for related risk factors. Fourth, all studies published by the same research team had to employ different patient data. Fifth, the studies included had to be reported in the English language. Studies with incomplete information and no related outcomes were excluded. The included studies had to be original studies, and all reviews, commentaries, case reports, abstracts, and meta-analyses were excluded.
Data extraction
Data from the included literature were independently extracted by two of the authors, and all conflicts were resolved by a third person. Extracted data included the last name of the first author, the year of publication, publication journal, sample size, demographic information (age, sex), etiologies, symptoms, imaging findings, laboratory parameters, and related prognostic indicators.
Statistical analysis
The ORs with their corresponding 95% CI for related risk factors were used to calculate the pooled estimates for the outcomes. To establish the appropriate weighting for each study, the SE for each logarithm OR (logOR) was calculated and was recognized as the estimated variance of the logOR. The generic inverse variance approach was applied for weighting. Statistical heterogeneity between the studies was quantified with the Q test (P>0.10), together with I2 values (I2<25%). When heterogeneity was present between studies, the fixed-effect and Laird random-effects model (D-L model) was applied to calculate the pooled estimate and its 95% CI (17). Sensitivity studies were performed by excluding individual studies to identify any study that significantly affected the overall estimates. Publication bias was represented as a funnel plot and further assessed by the Egger’s test (18). P<0.05 was considered as statistically significant. All statistical analysis was performed with Review Manager software (version 5.3.5; The Cochrane Collaboration) and STATA software (version 10.0; Stata Corporation). The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the inclusive studies. A study with a score of 7 or above is regarded as high quality.
Results
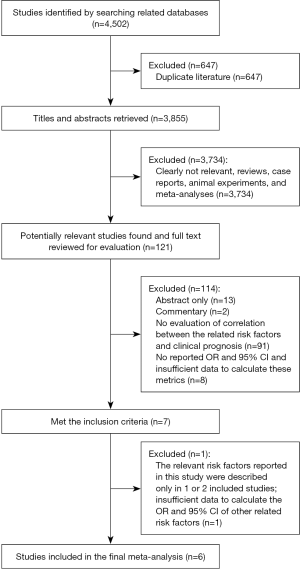
By querying the PubMed, EMBASE, Cochrane Library, and Web of Science databases updated up to November 2017, a total of 4,502 studies were identified. 647 studies were excluded due to duplication. Of the remaining 3,855 studies, 3,734 studies were excluded by thorough review of the titles and abstracts, including completely unrelated studies, reviews, case reports, animal experiments, and Meta-analyses. There were 121 studies remaining. These 121 articles were reviewed using the full text, 13 studies were only abstracts and 2 studies were commentaries, 91 studies did not evaluate the correlation between the related risk factors and clinical prognosis in patients with PRES, 8 studies did not report the OR and 95% CI for related risk factors or the data they provided were insufficient to calculate the corresponding OR and 95% CI. All these studies were excluded. A total of 7 studies met our inclusion criteria. However, 1 of these 7 studies was excluded because the relevant risk factors reported in this study were described only in one or two of the included studies and were not appropriate for consolidation, and there was insufficient information for the calculation of the OR and 95% CI of other related risk factors. Finally, there were only 6 studies (12,19-23) that were included in meta-analysis. All these studies were retrospective cohort studies with the sample size greater than 10 (Figure 1).
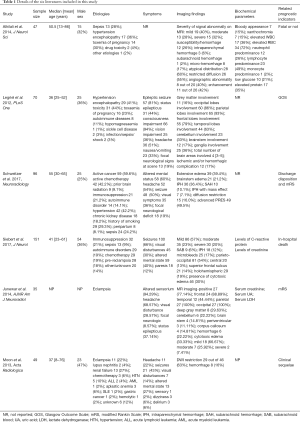
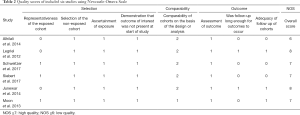
A total of 448 PRES patients were included in the 6 studies, and details of each study were provided in Table 1. In these studies, the impact of multiple conditions on clinical outcomes of PRES patients were analyzed including: hypertension, encephalopathy, toxemia of pregnancy (pre-eclampsia/eclampsia), autoimmune disease, sepsis, headache, seizure, status epilepticus, altered mental status, hemorrhage, diffusion restriction (cytotoxic edema), edema severity, cerebrospinal fluid biochemical parameters, altered coagulation, C-reactive protein. The OR and 95% CI of above risk factors were described in 5 of the 6 included studies. The remaining study provided the clinical and MRI data for each patient (23). The binary logistic regression was used to correlate the outcome with age, eclampsia, seizure, DWI restriction and hemorrhage, and their ORs and 95% CIs were calculated. The NOS was used to assess the quality of the included studies. Overall, two studies had a score of 8, three scored 7, one scored 6. Formal critical appraisal of the six studies indicated that the quality was high in five studies (NOS ≥7) and low in one study (NOS ≤6) (Table 2).
Full table
Full table
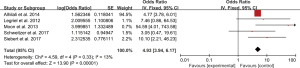
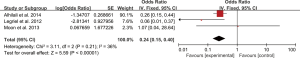
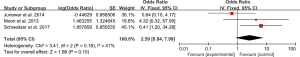
Five studies evaluated the impact of hemorrhage on the clinical outcome of PRES. No significant heterogeneity was observed between the 5 studies (Chi2=4.59, df=4, P=0.33; I2=13%). The pooled OR of the five studies under the fixed-effect model suggested that hemorrhage is associated with poor outcome in patients with PRES (pooled OR=4.93; 95% CI: 3.94–6.17; P<0.00001) (Figure 2). Sensitivity studies suggested that no individual study significantly affected the overall estimate of the meta-analysis. The Egger’s test (P=0.295) showed no significant publication bias in the included studies. The estimate from 3 pooled studies suggested that toxemia of pregnancy (pre-eclampsia/eclampsia) as the etiology of PRES may decrease the risk of poor outcome (pooled OR=0.24; 95% CI: 0.15–0.40; P<0.00001) (Figure 3). There was no significant heterogeneity between 3 studies (Chi2=3.11, df=2, P=0.21; I2=36%), and no significant publication bias was found (P=0.964). PRES patients with presence of diffusion restriction (cytotoxic edema) (pooled OR=2.59; 95%CI: 0.84–7.99; P=0.10) showed an unfavorable outcome in 3 pooled studies, but without statistical significance (Figure 4). No significant heterogeneity was identified in these 3 studies (Chi2=3.41, df=2, P=0.18; I2=41%). And as suggested by Egger’s test (P=0.316), no significant publication bias was evident. Of the 6 articles included, in addition to the risk factors mentioned above, hypertension encephalopathy, autoimmune disease, sepsis, moderate and severe edema, altered mental status, cerebrospinal fluid biochemical parameters, altered coagulation, C-reactive protein, higher serum creatinine, uric acid (UA), LDH levels, and highest glycaemia on day 1 were also related to the adverse outcome of PRES. However, these risk factors were evaluated in only 1 to 2 articles and could not be used for this meta-analysis.
Discussion
PRES is a neurotoxic syndrome and is usually considered to have a benign outcome. However, in previous studies, we frequently found long-term disability or death in the significant number of PRES patients (24). Previous studies of factors that affect the prognosis of PRES are few in number and the results are variable. Data on hemorrhage, toxemia of pregnancy (pre-eclampsia/eclampsia), diffusion restriction (cytotoxic edema) and association of these factors with poor outcomes of patients with PRES is limited. We reviewed and evaluated the related risk factors for poor outcomes in PRES systematically through meta-analysis. In this meta-analysis, hemorrhage was associated with high risk of poor outcome in patients with PRES. Toxemia of pregnancy (pre-eclampsia/eclampsia) may be associated with improved outcomes in PRES patients. There was a trend towards worse outcomes in patients with diffusion restriction (cytotoxic edema), but that did not reach statistical significance.
Most of the PRES patients showed symmetric vasogenic edema in bilateral posterior cerebral circulation territories on MRI imaging. However, multiple studies showed that the anterior circulation territory and infratentorial (brainstem, cerebellum) regions may also be involved. Atypical imaging findings such as hemorrhage, cytotoxic edema/infarction, abnormal enhancement, may occur in PRES patients (5,6). About 10–32% PRES cases were found to have intracranial hemorrhage (2,25,26). These PRES-associated intracranial hemorrhages included intraparenchymal hemorrhages, subarachnoid hemorrhages (SAH) or both. In a retrospective study including 23 patients with PRES-associated intracranial hemorrhage performed by Hefzy (25), hemorrhage was divided into 3 types including minute focal hemorrhage (<5 mm), sulcal SAH and focal hematoma, and these three types of hemorrhages were found to occur with similar frequency. With increased application of susceptibility weighted imaging (SWI), some studies showed that PRES patients had a high rate of microhemorrhage (MH) (27). This may be due to the higher sensitivity of SWI for intracranial hemorrhages which made it easier to identify hemorrhages. In Alhilali’s study cohort, MH occurred in 8 patients, accounting for 67% of the patients with hemorrhage, demonstrating that in this study MH is the most common type of hemorrhage (19).
The imaging features of hemorrhage in patients with PRES have been well described in previous studies. However, in most studies, the relationship between hemorrhage and prognosis of PRES patients, and whether hemorrhage can predict an unfavorable outcome have not been formally investigated. Of the 7 patients with intracranial hemorrhage reported by Aranas et al. (28) in 2009, only two patients had good functional outcomes in PRES. Moon et al. (23) investigated the association between clinical or MRI findings and the prognosis of variant and classical types of PRES in a retrospective study. Their results showed that most patients with clinical or structural sequelae had hemorrhage or irreversible lesions in both types of PRES patients. A prospective study of 25 patients with PRES showed that 3 patients with poor outcomes had coexistent multiple diseases or intracranial hemorrhages (29). The results of the above studies are consistent with our analysis that hemorrhage predicts a poor outcome for PRES. However, no significant correlation between hemorrhage and adverse outcomes in patients with PRES has been reported (30). Some investigators believe that a small amount of hemorrhage does not affect the outcome, whereas multiple or massive hemorrhages may be one of the predictors for poor outcome in PRES. The relationship between hemorrhage and the prognosis of PRES remains uncertain, as there is no consistent conclusion between the different studies. Most of the relevant studies were single center studies, and the sample size, patient population, and risk factors for single center studies are limited. As discussed above, the use of SWI may have an impact on the detection of hemorrhage. The heterogeneity of prognostic criteria may also have an impact on the outcome of the study. In prior studies, modified Rankin scale (mRS), Glasgow Outcome Scale (GOS) and hospital discharge disposition were used to assess prognosis of patients with PRES. In addition, the impact of hemorrhage types, location, and severity on the clinical and prognostic of PRES have not been well described. In five of the six studies we have included, the impact of hemorrhage on the clinical outcome of PRES was evaluated. Although there were multiple manifestations of hemorrhage on imaging of PRES, only one of them has analyzed the effects of intracerebral hemorrhage and SAH on its prognosis (22). As far as we know, there were three studies (19,21,23) which classified the types of hemorrhages in patients with PRES, these were collectively referred to as hemorrhages in assessing the impact on prognosis. Most studies did not classify hemorrhage on imaging in PRES patients, collectively termed as hemorrhage. Therefore, in this meta-analysis, we could not determine which kind of hemorrhage in PRES patients had the worst prognosis. We have to admit that this is a limitation of this study, and which might be a research focus in the future. Some studies have shown that the incidence of hemorrhage varies in PRES patients under different etiological conditions. A study by Hefzy et al. (25) showed that PRES related-hemorrhage occurred with high incidence in the context of immunosuppression and with lower incidence in eclampsia. Hemorrhage may represent a direct risk factor for poor outcome in PRES, or it may represent secondary sequalae in more severe cases of PRES. This significance of hemorrhage in this setting requires further investigation. Therefore, a large, multicenter, prospective or retrospective study is needed to thoroughly evaluate these questions.
The mechanism of hemorrhage in patients with PRES remains unknown. Some researchers believed that PRES-related SAH may be associated with severe hypertension and cerebral autoregulation loss, while multifocal hemorrhage may be caused by post-ischemic reperfusion injury (30). However, a recent study showed that PRES related hemorrhages were not associated with moderate or severe hypertension, and various hemorrhage types appear to occur with similar frequency in the different PRES toxicity-associated conditions (24). In previous studies of PRES patients, about two thirds of patients experienced an elevation of blood pressure when symptoms occurred. PRES can be considered a consequence of hypertension crisis. The clinical and imaging manifestations of hypertensive encephalopathy may disappear after timely treatment, and many scholars believe that it is a form of reversible posterior encephalopathy syndrome, which occurs in patients with severe hypertension. Both disorders have similar neurological symptoms and imaging findings in the course of the disease, suggesting that there may have some common pathogenesis between them. Therefore, hypertension has been emphasized as a common feature in all PRES related conditions in the past period of time. The elevation of systemic blood pressure beyond the upper limit of cerebrovascular autoregulation leads to the transient loss of the ability of cerebrovascular autoregulation and subsequent hyperperfusion, endothelial injury and breakdown of the blood-brain barrier, eventually resulting in vasogenic edema. At present, there are few studies on the correlation between blood pressure and imaging findings of PRES. In our previous series of studies, we found no significant correlation between hypertension and the degree and location of brain edema (31). Some scholars believe that the distribution of cerebral edema is related to the severity of elevated blood pressure (32). While mild hypertension only causes edema in the supratentorial area, severe hypertension can cause wider supratentorial brain edema. Edema may also involve the brain stem, cerebellum and basal ganglia (32). Some studies also showed that there was a certain correlation between higher edema grade and higher SBP, however there was no statistical difference (2). In our recent animal trials, we found that brain edema lags behind blood pressure fluctuations. With increasing awareness of the syndrome, most researchers have found that PRES can also occur in patients with normal or only slightly elevated blood pressure. Elevated blood pressure may be an important cause of brain edema, but it is not a necessary condition. The role of blood pressure in the formation of cerebral edema in patients with PRES remains to be further explored. Now PRES is generally considered to be a systemic toxic process, and its pathogenesis is still controversial. Currently, three main theories have been proposed, including vasoconstriction/hypoperfusion, hypertension/reperfusion, and endothelial dysfunction theory (33-35). PRES-associated hemorrhage usually occurs in areas of cerebral edema, and is associated with edema severity and development of cytotoxic edema. Either the hyperperfusion induced by the loss of cerebral autoregulation or the damage of blood-brain barrier (BBB) caused by endothelial cell injury can lead to the increase of capillary permeability. Plasma and erythrocytes exude into the subarachnoid space and brain parenchyma resulting in SAH and punctate hemorrhages. When a sudden rise of blood pressure occurs, increased intravascular pressure can lead to the rupture of the damaged vessel wall and hemorrhage. In PRES patients, the hemorrhage in the region of cerebral edema is mostly characterized by stasis and punctate hemorrhages. Hemorrhage on SWI is manifest as multiple or scattered low signal intensity foci in the edematous region. We believe that endothelial cell injury may play an important role in PRES and PRES-related hemorrhage.
Although vasogenic edema is the typical imaging feature of PRES patients, conversion to cytotoxic edema has also been reported in previous studies (36-38). In the previously reported studies, approximately 10–33% of PRES patients developed cytotoxic edema (12,14,27,38,39). This may be due to the lack of timely treatment, resulting in persistent hyperperfusion, leading to microcirculatory disturbances in the compromised tissue, which eventually develop ischemia, edema, and infarction. Studies have shown that cytotoxic edema is related to the overall extent of cerebral edema, and ischemia is more likely to occur in a large region of cerebral edema (40,41). This may be related to the local hypoperfusion, the decrease of cerebral blood flow and the increase of tissue pressure caused by the mass effect of extensive cerebral edema (2). In a study carried out by Junewar (12), there is a higher incidence of cytotoxic edema in PRES patients with eclampsia, which may be related to reactive vasoconstriction. Previous findings are inconclusive regarding the relationship between diffusion restriction (cytotoxic edema) and clinical prognosis of PRES. A study performed by Covarrubias et al. (42) showed that diffusion restriction may represent the earliest sign of irreversible lesions. Studies have also shown that there is no significant correlation between diffusion restriction and mortality (19). In some studies diffusion restriction completely resolved during follow-up (14). The reasons for these inconsistencies may be due to differences in sample size, research population, and prognostic criteria. Our analysis showed that diffusion restriction is associated with adverse outcome in patients with PRES, which was not statistically significant. Therefore, multicenter studies with standardized prognostic criteria are needed to investigate the relationship between diffusion restriction and clinical prognosis in patients with PRES.
The degree of cerebral edema in patients with PRES varies greatly, from small cortical, subcortical foci to large fused lesions. With the advancement of the research in PRES and rapid development of imaging techniques, the evaluation criteria for the degree of brain edema in PRES are constantly updated. In 2000 (43), the grade of PRES brain edema was first presented according to the scope and occupying effect of edema, which was divided into the 0–3 grade. In 2006, Bartynski et al. (44) also proposed a grading basis for the degree of cerebral edema, which was classified into five grades according to the depth of edema and the occupying effect of the most affected area. In 2016, Karia et al. (45) classified the degree of brain edema into 4 grades according to the location, the fusion and the occupying effect of the edema. Only one of the six studies included indicated that moderate or severe brain edema was associated with death in patients with PRES. In the future study of the relationship between the degree of cerebral edema and the prognosis of PRES, a unified standard of the degree of cerebral edema is essential.
Our analysis showed that toxemia of pregnancy (pre-eclampsia/eclampsia) may be associated with reduced risk of adverse outcome in patients with PRES. All PRES patients with eclampsia had reversible PRES lesions in a study conducted by Pande et al. (14) in 2006. And this may reflect a relatively benign pathophysiological process. Previous studies have also demonstrated that PRES patients with preeclampsia-eclampsia have less severe imaging findings and clinical symptoms (46). The cause of this phenomenon remains unknown. This may be due to a fundamental difference in pathophysiological mechanisms or due to more careful monitoring of the symptoms and signs of eclampsia or pre-eclampsia in perinatal women. In addition, Alhilali et al. (19) suggest that rapid reversal of blood pressure caused by emergency delivery of pregnant women may also improve outcomes. However, other studies that indicate that incomplete recovery occur more frequently in PRES patients with eclampsia than without eclampsia (47). This may be related to extra-neurological complications as well as neurological complications, such as HELLP syndrome, ischemia and hemorrhage.
After a series of analysis, no significant heterogeneity was found among the included studies, and no publication bias was identified. However, limitations of the current meta-analysis should be acknowledged. First, only 6 studies were included in this meta-analysis, and only the relationships between hemorrhage, diffusion restriction (cytotoxic edema), toxemia of pregnancy (pre-eclampsia/eclampsia) and PRES outcomes were assessed. We have performed a comprehensive search and screening using the PubMed, EMBASE, Cochrane Library, and Web of Science databases. However, at present, there are very few studies investigating the prognosis of PRES. Second, the 6 included studies were retrospective cohort studies, with the typical associated limitations of retrospective studies, such as selection and information bias. Third, the prognostic evaluation criteria were not uniform. mRS, GOS and hospital discharge disposition were used in the different studies, respectively. Although these evaluation criteria use different parameters, they all reflect the clinical functional prognosis of the patient. Therefore, we believe that the poor clinical prognosis of the patients as reflected in these criteria can be considered consistent. It is undeniable that more consistent and comprehensive evaluation criteria for PRES prognostic factors are necessary. Fourth, SWI is more sensitive for detection of hemorrhage, yet not all the included studies used this sequence to detect intracranial bleeding. This may also influence the results of the study. Fifth, we only evaluated the effect of hemorrhage on the prognosis of PRES, and could not determine which kind of hemorrhage in PRES patients had been also the worst prognosis. Sixth, inability to assess the degree of vasogenic edema as a potential risk factor limits the study. More studies are needed to investigate whether hemorrhage, cytotoxic edema, and pre-eclampsia/eclampsia are associated with adverse outcome of PRES and to evaluate the impact of other related risk factors on PRES. More consistent and comprehensive evaluation criteria for PRES prognostic factors are necessary. SWI and DWI should be used as routine sequences in PRES imaging protocols. This will allow for more accurate correlation between microhemorrhages, cytotoxic edema, and PRES outcomes and will further contribute to the study of the mechanisms of PRES.
Conclusions
The results of this meta-analysis suggest that hemorrhage is associated with poor outcome in PRES. Toxemia of pregnancy (pre-eclampsia/eclampsia) is associated with an improved outcome in PRES patients. Diffusion restriction (cytotoxic edema) may indicate a poor outcome, although this results did not reach statistical significance. Larger studies are warranted to evaluate the detrimental effects of hemorrhage, cytotoxic edema, pre-eclampsia/eclampsia and other related risk factors in PRES patients.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (Grant No. 81471645), Shandong Provincial Key Research & Development Project (Grant No. 2015GSF118185).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This project was performed in compliance with institutional guidelines and was approved by the Institutional Review Board of Yantai Yuhuangding Hospital (No. YYY Ethics-2016-03).
References
- Gao B, Lyu C, Lerner A. McKinney. Controversy of posterior reversible encephalopathy syndrome: what we have learnt in the last 20 years? J Neurol Neurosurg Psychiatry 2018;89:14-20. [Crossref] [PubMed]
- Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol 2012;259:155-64. [Crossref] [PubMed]
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914-25. [Crossref] [PubMed]
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500. [Crossref] [PubMed]
- Singh RR, Ozyilmaz N, Waller S. U-King-Im JM, Lim M, Siddiqui A, Sinha MD. A study on clinical and radiological features and outcome in patients with posterior reversible encephalopathy syndrome (PRES). Eur J Pediatr 2014;173:1225-31. [Crossref] [PubMed]
- Pereira PR, Pinho J, Rodrigues M, Rocha J, Sousa F, Amorim J, Ribeiro M, Rocha J, Ferreira C. Clinical, imagiological and etiological spectrum of posterior reversible encephalopathy syndrome. Arq Neuropsiquiatr 2015;73:36-40. [Crossref] [PubMed]
- Pavlidou E, Pavlou E, Anastasiou A, Pana Z, Tsotoulidou V, Kinali M, Hatzipantelis E. Posterior reversible encephalopathy syndrome after intrathecal methotrexate infusion: a case report and literature update. Quant imaging Med Surg 2016;6:605-11. [Crossref] [PubMed]
- Zhang L, Wang Y, Shi L, Cao J, Li Z, Wáng YX. Late postpartum eclampsia complicated with posterior reversible encephalopathy syndrome: a case report and a literature review. Quant Imaging Med Surg 2015;5:909-16. [PubMed]
- Gungor S, Kilic B, Tabel Y, Selimoqlu A, Ozqen U, Yilmaz S. Clinical and imaging findings in childhood posterior reversible encephalopathy syndrome. Iran J Child Neurol 2018;12:16-25. [PubMed]
- Akins PT, Axelrod Y, Silverthorn JW, Guppy K, Banerijee A, Hawk MW. Management and outcome of malignant posterior revesible encephalopathy syndrome. Clin Neurol Neurosurg 2014;125:52-7. [Crossref] [PubMed]
- Faille LD, Fieuws S, Van Paesschen W. Clinical predictors and differential diagnosis of posterior reversible encephalopathy syndrome. Acta Neurol Belg 2017;117:469-75. [Crossref] [PubMed]
- Junewar V, Verma R, Sankhwar PL, Garq RK, Singh MK, Malhotra HS, Sharma PK, Parihar A. Neuroimaging features and predictors of outcome in eclamptic encephalopathy: a prospective observational study. AJNR Am J Neuroradiol 2014;35:1728-34. [Crossref] [PubMed]
- Pirker A, Kramer L, Voller B, Loader B, Auff E, Prayer D. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol 2011;32:527-31. [Crossref] [PubMed]
- Pande AR, Ando K, Ishikur A R, Naqami Y, Takada Y, Wada A, Watanabe Y, Miki Y, Uchino A, Nakao N. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med 2006;24:659-68. [Crossref] [PubMed]
- Liman TG, Bohner G, Endres M, Siebert E. Discharge status and in-hospital mortality in posterior reversible encephalopathy syndrome. Acta Neurol Scand 2014;130:34-9. [Crossref] [PubMed]
- Gao B, Lerner A, Law M. The clinical outcome of posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2016;37:E55-6. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Alhilali LM, Reynolds AR, Fakhran S. A multi-disciplinary model of risk factors for fatal outcome in posterior reversible encephalopathy syndrome. J Neurol Sci 2014;347:59-65. [Crossref] [PubMed]
- Legriel S, Schraub O, Azoulay E, Hantson P, Maqalhaes E, Coquet I, Bretonniere C, Gilhodes O, Anguel N, Megarbane B. Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One 2012;7:e44534. [Crossref] [PubMed]
- Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, Knobel A, Navi BB, Young RJ, Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology 2017;59:379-86. [Crossref] [PubMed]
- Siebert E, Bohenr G, Liebig T, Endres M, Liman TG. Factors associated with fatal outcome in posterior reversible encephalopathy syndrome: a retrospective analysis of the Berlin PRES study. J Neurol 2017;264:237-42. [Crossref] [PubMed]
- Moon SN, Jeon SJ, Choi SS, Song CJ, Chung GH, Yu IK, Kim DH. Can clinical and MRI findings predict the prognosis of variant and classical type of posterior reversible encephalopathy syndrome (PRES)? Acta Radiologica 2013;54:1182-90. [Crossref] [PubMed]
- Hinduja A, Habetz K, Raina S, Ramakrishnaiah R, Fitzgerald RT. Predictors of poor outcome in patients with posterior reversible encephalopathy syndrome. Int J Neurosci 2017;127:135-44. [Crossref] [PubMed]
- Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol 2009;30:1371-9. [Crossref] [PubMed]
- Sharma A, Whitesell RT, Moran KJ. Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology 2010;52:855-63. [Crossref] [PubMed]
- McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. Am J Neuroradiol 2012;33:896-903. [Crossref] [PubMed]
- Aranas RM, Prabhakaran S, Lee VH. Posterior reversible encephalopathy syndrome associated with hemorrhage. Neurocrit Care 2009;10:306-12. [Crossref] [PubMed]
- Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry 2010;81:773-7. [Crossref] [PubMed]
- Doss-Esper CE, Singhal AB, Smith MS, Henderson GV. Reversible posterior leukoencephalopathy, cerebral vasoconstriction, and strokes after intravenous immune globulin therapy in Guillain-Barre syndrome. J Neuroimaging 2005;15:188-92. [PubMed]
- Bo G, Hui L, Feng-Li L, Cui L. Relationships between edema degree and clinical and biochemical parameters in posterior reversible encephalopathy syndrome: a preliminary study. Acta Neurol Belg 2012;112:281-5. [Crossref] [PubMed]
- Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000;217:371-6. [Crossref] [PubMed]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043-9. [Crossref] [PubMed]
- Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses 2014;82:619-22. [Crossref] [PubMed]
- Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, Magliulo G. Posterior reversible encephalopathy syndrome--Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2015;14:830-6. [Crossref] [PubMed]
- Gao B, Liu FL, Zhao B. Association of degree and type of edema in posterior reversible encephalopathy syndrome with serum lactate dehydrogenase level: initial experience. Eur J Radiol 2012;81:2844-7. [Crossref] [PubMed]
- Gao B, Lv C. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia: beyond it. Am J Obstet Gynecol 2014;211:83-4. [Crossref] [PubMed]
- Gao B, Yu BX, Li RS, Zhang G, Xie HZ, Liu FL, Lv C. Cytotoxic edema in posterior reversible encephalopathy syndrome: correlation of MRI features with serum albumin levels. AJNR Am J Neuroradiol 2015;36:1884-9. [Crossref] [PubMed]
- Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol 2004;190:714-20. [Crossref] [PubMed]
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007;189:904-12. [Crossref] [PubMed]
- Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009;51:373-83. [Crossref] [PubMed]
- Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol 2002;23:1038-48. [PubMed]
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detecion of cortical and subcortical lesions. AJNR Am J Neuroradiol 2000;21:1199-206. [PubMed]
- Bartynski WS, Boradman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 2006;27:2179-90. [PubMed]
- Karia SJ, Rykken JB, Mckinney ZJ, Zhang L, McKinney AM. Utility and significance of gadolinium-based contrast enhancement in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2016;37:415-22. [Crossref] [PubMed]
- Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol 2012;19:935-43. [Crossref] [PubMed]
- Zeeman GG. Neurologic complications of pre-eclampsia. Semin Perinatol 2009;33:166-72. [Crossref] [PubMed]