Spleen and splenic vascular involvement in acute pancreatitis: an MRI study
Introduction
Acute pancreatitis (AP) is an inflammatory disorder that leads to a wide range of local and systemic pathophysiological changes, as well as protean clinical manifestations and prognosis (1,2). In the development and progression of AP, especially in severe AP, it is believed that a complex cascade of inflammatory responses and subsequent immune abnormality play important roles (3,4). The spleen, as an important immune organ of the human body, can produce many immune factors and immunocytes that are important for immunological regulation in AP, but it is also injured in inflammatory reactions and could intensify the severity of the human body’s condition (5).
The splenic abnormalities in AP reported by radiologists were mainly through CT (6-8). However, CT has some limitations; its lack of sensitivity to edematous and mild AP and its radiation and potential iodine toxicity could produce injury to the human body (9-11). Magnetic resonance imaging (MRI) is sensitive to the mild and edematous AP and clearly shows necrotic areas even without enhancement and assesses the inflammatory changes accurately, offering complementary information for CT in the assessment of pancreatic and peripancreatic changes. MRI has become a more and more important tool in evaluating the pancreas and peripancreas changes (11-13). Furthermore, the intravoxel incoherent motion (IVIM) MRI can help to assess the functional properties of tissues, reflecting the random microscopic motion that occurs in voxels on the MR images of water molecule (either intracellular or extracellular) and the microcirculation of blood. Quantitative parameters could separately reflect the tissue diffusivity and tissue microcapillary perfusion to be estimated (14-16). IVIM signal is modeled with the equation:
SI(b) = SI0[(1−PF)·exp(−b·Dslow) + PF·exp(−b·Dfast)] [1]
where SI(b) and SI0 denote the signal intensity acquired with the b-factor value of b and b =0 s/mm2, respectively. Dslow (D) is the true diffusion coefficient that represents the pure molecular diffusion (slow component of diffusion), Dfast (D*) is the pseudo-diffusion coefficient representing the incoherent microcirculation within the voxel (perfusion-related diffusion, or fast component of diffusion), and perfusion fraction (PF) (f) is the fraction of the pseudo-diffusion linked to microcirculation. These characteristics may detect and characterize the tissue changes caused by disease (14-16).
The aim of this retrospective study was to investigate the prevalence of spleen and splenic vascular involvement in AP using MRI and their correlations with the severity of AP in the clinical and imaging setting, and to investigate the changes of the spleen in AP using IVIM.
Methods
Ethics statement
The Institutional Review Board and Ethics Committee of our institute approved this study and waived patients’ informed consent because it was a retrospective study.
Patient selection
Patients with AP admitted to our institution from January 2014 to December 2016 were included. The inclusion criteria were as follows: (I) in-patient; (II) acute onset of abdominal pain; (III) at least 3-fold elevated amylase or lipase; (IV) pancreatitis at first onset; and (V) abdominal MRI examination within 1 week of the pancreatitis onset. The exclusion criteria were as follows: (I) inability to cooperate when performing MRI; (II) a history of chronic pancreatitis and previous episodes of AP; (III) AP resulting from pancreatic carcinoma; (IV) hypoproteinemia or peritoneal/retroperitoneal tumors; (V) presence of other diseases with splenic involvement (including chronic liver disease, cirrhosis, hematological and immune system diseases); (VI) splenic disease (including splenic inflammatory, tumor, parasitosis) and (VII) splenectomy.
A total of 331 patients with AP met the inclusion criteria, and 92 of these patients met the exclusion criteria (5 met condition I; 48 met condition II; 4 met condition III; 5 met condition IV; 16 met condition V; 8 met condition VI; 6 met condition VII) were not included in the study. The final study consisted of 239 consecutive patients.
Thirty-five continuous subjects without pancreatic and splenic disorders visible on MRI between January 2016 and December 2016 were enrolled as the control group. The subjects had no abdominal symptoms or signs and nothing that could cause splenic abnormalities. MRI showed 12 patients without abnormal findings in the abdomen, 15 patients with hepatic or renal cysts, and eight patients with hepatic hemangioma visualized on MRI.
MRI technique
All of the MR examinations were performed on a 3.0-T system (MR750, GE Medical Systems, Waukesha, WI, USA). The sequences included two-dimensional coronal and axial single-shot fast spin-echo (SSFSE) T2-weighted, axial fast-recovery fast spin-echo (FRFSE) T2-weighted fat suppressed, three-dimensional (3D) liver acquisition with volume acceleration-flexible (3D LAVA-flex) before enhancement, which can produce fat suppressed T1-weighted imaging as well as T1-weighted in-phase and out-phase images (17), 3D LAVA-flex dynamic contrast-enhanced with fat-suppressed and SSFSE radial series slab MR cholangiopancreatography (MRCP).
The coronal SSFSE T2-weighted MR images were obtained in two or more breath-holds with the following parameters: repetition time (TR)/echo time (TE) =4,500–6,000 ms/90–120 ms; section thickness =5 mm; intersection gap =1; matrix =384×256; and field of view (FOV) =36 cm × 36 cm. The axial SSFSE T2-weighted images (T2WI) were obtained in one or two breath-holds with the following parameters: TR/TE =4,500–6,000 ms/90–120 ms; section thickness =6 mm; intersection gap =1; matrix =320×256; and FOV =34 cm × 34 cm. The axial FRFSE T2-weighted with fat suppressed images were obtained with the following parameters: TR/TE =2,500–3,000 ms/90–110 ms; section thickness =6.0 mm; intersection gap =1; matrix =384×384; and FOV =34 cm × 34 cm. SSFSE radial oblique slabs were obtained for MRCP with the following parameters: TR/TE =4,000–5,000 ms/900–1,000 ms; fat saturation; section thickness =50 mm; matrix =384×256; and FOV=34 cm × 34 cm. The axial LAVA MR images were obtained with TR/TE =3.6–4.4 ms/1.7–1.9 ms; flip angle =12°; matrix =224×192; section thickness =5.2 mm; intersection gap =0; FOV =36 cm × 36 cm; and NEX =0.75. 3D LAVA was obtained before and after enhancement. For dynamic enhancement, 20 mL of gadolinium (Magnevist; Schering Guangzhou, China) was administered intravenously with a pressure injector (Spectris MR Injection System, Medrad, Inc, USA) at 2–3 mL/s, followed by a 20-mL saline solution flush. For the dynamic contrast-enhancement, the scanning for the early hepatic arterial, hepatic arterial, venous, and delayed phases were set at 16, 30, 60, and 120 s after the injection of the contrast medium, respectively.
The IVIM DW imaging sequence was based on a single-shot DW spin echo planar imaging fat-suppressed sequence, the specific parameters were as follows: b-factor values (0, 20, 50, 80, 100, 300, 500, and 800 s/mm2); TR/TE =4,000–4,500 ms/90–100 ms; matrix =160×192; FOV =34 cm × 34 cm; section thickness =6 mm; and intersection gap =2 mm. The original IVIM images were loaded to a post-processing workstation (GE Advanced Workstation v.4.4-09) and the IVIM data were evaluated by a standard software package (MADC); the ADC, D, D*, and f maps were generated by an automatic pixel-wise analysis on the GE workstation. The IVIM signal attenuation was modeled according to Eq [1]. D was obtained by using a least-squares linear fitting of the logarithmized image intensity at b values greater than 200 s/mm2 to a linear equation; the fitted curve was then extrapolated to obtain an intercept at b =0. The estimate of f was obtained by the ratio between this intercept and SI0; finally, the obtained D and f were substituted into equation 1 {Eq [1]} and were nonlinear leastsquares fitted against all b values to estimate D* using the Levenberg-Marquardt algorithm.
In each spleen, the region of interest (ROI) was set as 50±5 mm2 and six ROIs were manually drawn within the middle of the spleen in three slices at the level of the hilus carefully excluding vasculature (16). The ROIs were automatically copied to the D, D*, and f maps, the mean value of each ROI was recorded. Mean value of the three parameters were calculated as the final results of statistical analyses.
MR image review
The original MRI data were loaded onto a workstation (GE, AW4.4) for observation and measurement. The MR images were reviewed by two observers (with at least three years of experience in abdominal MRI), who were blinded to the laboratory data and clinical outcomes. Any discrepancies between the two readers were discussed until reaching a consensus.
AP was categorized as edematous or necrotizing according to the MRI findings (18,19). The severity of AP on MRI was graded as mild (0–3 points), moderate (4–6 points) and severe (7–10 points) according to the MRI severity index (MRSI) (20). In clinical practice, AP was graded as mild, moderately severe, and severe AP based on the New Revised Classification of AP 2012 (21).
To describe the splenic vascular patterns and splenic morphology on MRI, the detected characteristics and measured dimensions are outlined below. The vascular involvement includes pseudoaneurysm which was defined as a cavity communicated with the splenic artery that might have mural thrombosis; on the enhanced images, the enhancement of cavity corresponds with the artery and the mural thrombus was non-enhanced (22,23); arteritis or arterial wall invasion defined as a linear or patchy high signal consistent with the running of the splenic artery or even the loss of a normal vascular flowing void effect on T2WI with fat suppression. The blurred edge of the involved artery or poor enhancement of the involved segment on contrast-enhanced arterial phase images (22,24); Phlebitis or venous wall invasion has also been defined as a linear or patchy high signal consistent with the running of the splenic vein and even the loss of the normal vascular flowing void effect on T2WI and T2WI with fat suppression, the unsharpness of the blood-vessel-edge or poor enhancement of the involved segment on contrast-enhanced venous phase images; additionally, it might be complicated by local venous thrombosis, which showed an intravenous filling defect or the vein was undetectable on the enhanced venous phase images (22-24). Splenomegaly or splenic enlargement is the most common condition associated with diseases of the spleen, “splenic index” (product of length, depth and width), splenic length and the sum of volumes of consecutive scan slices have all been used to diagnose the splenomegaly (25). The best indicator of splenic changes over time is splenic volume, however, it is time-consuming and impractical in clinical routine (25-27). Correlations of splenic length, width and thickness with the splenic volume had been studied and we found that the splenic length and width correlate well with splenic volume (26,27), a splenic length of 9.76 cm as an easier and simplified method can be used to diagnose the splenomegaly (26). In our study, Splenomegaly was defined as splenic length >9.76 cm with this easier and simplified way. The splenic length was obtained by multiplying the number of sections where the spleen was visualized by the thickness of the sections (26). In this study, the splenic length was obtained by using this way on the delayed phase of dynamic-enhancement, which had a single breath-hold that could exclude the movement artifacts and which had no intersection gap that was convenient to calculate. For example, if the spleen was seen in 20 contiguous cross-sectional images with 5-mm thickness on the delayed phase, the length was recorded as 10 cm.
Statistical analysis
The MRSI scores and IVIM parameters (D, D*, f) of spleen were expressed as the average of the two readers. Any discrepancies between the two readers were discussed until reaching a consensus.
The continuous variables were expressed as mean ± SD and range. Chi-squared test or Fisher’s exact test were used to evaluate the differences in the prevalence of splenic vascular involvement and splenomegaly among mild, moderate/moderately severe, and severe AP (based on the MRSI and the New Revised Classification of AP 2012), between edematous and necrotizing AP. Mann-Whitney U-test was used to analyze the differences in the splenic D, D*, and f values between the AP and control groups, and the differences in the splenic D, D*, and f values between AP patients with and without splenomegaly.
Data analysis was performed using SPSS for Windows version 13.0 (SPSS, Inc, Chicago, IL, USA). P values <0.05 were considered to be significant.
Results
Demographical characteristics
Thirty-five subjects in the control group included 15 males and 20 females, with an average age of 48.9±16.9 years. A total of 239 patients with AP included 119 women and 120 men with an average age of 51.8±14.6 years (range, 10–81 years). There were no statistically significant differences in age (t=1.058, P=0.291) or the sex ratio (χ2=0.660, P=0.416) between the AP and control groups.
In the 239 cases with AP, the etiology of AP was biliary in 117 (49.0%) cases, hyperlipidemia related in 57 (23.8%) cases, alcohol related in 19 (7.9%) cases, surgery related in 3 (1.3%) cases, and unknown in 43 (18.0%) cases.
MRI findings of AP
Of the 239 patients with AP, 187 patients (78.2%) had edematous AP, whereas 52 patients (21.8%) had necrotizing AP. The mean MRSI score was 3.52±1.68 (range, 0–10). According to the MRSI, the mild, moderate, and severe AP were 42.7% (102/239), 51.9% (124/239), and 5.4% (13/239), respectively.
Splenic morphology and vascular involvement on MRI
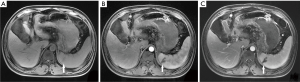
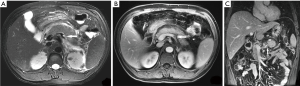
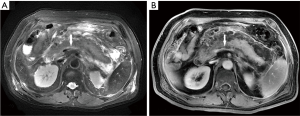
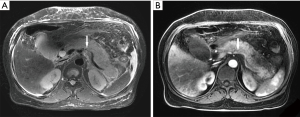
Of the 239 patients with AP, 10.5% (25/239) of patients had splenic vascular involvement, 16.7% (40/239) of patients had splenomegaly, and 0.4% (1/239) of patients had splenic infarction (Figure 1). The splenic vascular involvement included vein thrombosis (4.2%, 10/239) (Figure 2), phlebitis (7.5%, 18/239) (Figure 3), and arteritis (4.2%, 10/239) (Figure 4).
Correlations of prevalence of splenic vascular involvement and splenomegaly with the severity of AP based on MRSI
Of the 239 patients with AP, based on the MRSI, the prevalence of splenic vascular involvement in mild, moderate, and severe AP were, respectively, 2.0%, 11.3%, and 69.2% (χ2=36.442, P<0.001). The prevalence of splenomegaly in mild, moderate, and severe AP were, 13.7%, 18.6%, and 23.1% (χ2=1.561, P=0.455), respectively. Additionally, the prevalence of splenic vascular involvement was 3.7% and 34.6% (χ2=41.402, P=0.000) in edematous AP and necrotizing AP, respectively. The prevalence of splenomegaly in edematous AP and necrotizing AP were 15.3% and 23.3% (χ2=1.599, P=0.206).
Correlations of prevalence of splenic vascular involvement and splenomegaly with the severity classification in New Revised Classification of AP 2012
Among the 239 patients with AP, according to the New Revised Classification of AP 2012, the mild, moderately severe, and severe AP were 48.1% (115/239), 42.7% (102/239), and 9.2% (22/239), respectively. The prevalence of splenic vascular involvement in mild, moderately severe, and severe AP were 2.5%, 15.3%, and 31.8% (χ2=19.577, P=0.000), respectively. The prevalence of splenomegaly in mild, moderately severe and severe AP were, respectively, 14.7%, 18.6% and 18.2% (χ2=0.719, P=0.760).
IVIM parameters (D, D*, f) of spleen in the control and AP groups, and the parameters in AP patients with splenomegaly and nonsplenomegaly
AP was divided into two groups: AP without splenomegaly [AP (no SP)] and AP with splenomegaly [AP (with SP)]. The splenic f value was higher in the AP group than in the control group (P<0.05). Additionally, the splenic f value in the AP (with SP) group was higher than that in the AP (no SP) group (P<0.05) (Figure 5).
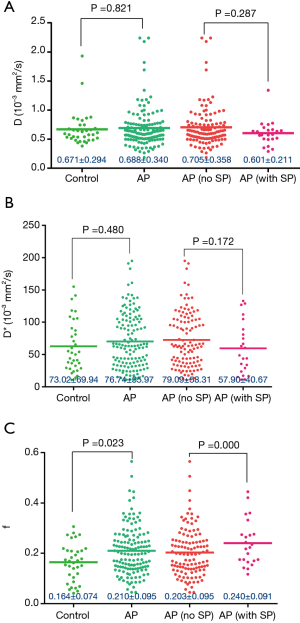
All patients during the hospital stay had good recovery and were discharged after treatment. No patient died.
Discussion
The prevalence of splenic vascular involvement was positively correlated with the severity of AP based on both the MRSI and the New Revised Classification of AP 2012. On IVIM, the splenic f value was higher in AP patients than that in the control group; in AP patients, the splenic f value in patients with splenomegaly was higher than in those without splenomegaly. The splenic perfusion fraction increased in AP patients, especially in those with splenomegaly. Our results indicated that the splenic vascular involvement in AP may help to evaluate the severity of AP in the clinical and imaging setting. Quantitative analysis of the spleen with IVIM showed that AP may cause perfusion changes in splenic tissue, especially in those with splenomegaly.
With the close anatomic relationship, inflammatory exudates in AP might directly affect the spleen and splenic vasculature (8,25). The prevalence of splenic vein thrombosis in pancreatitis varies in different studies from 1% to 24% depending on the study population and imaging techniques been used and the study time (23,28-30). In our study, the prevalence of splenic vein thrombosis was 4.5%, which was higher than 1.2% (30/2,454) in the study of Harris et al. (28), who used CT to evaluate the splenic vein thrombosis. Our imaging technique was MRI which had higher tissue resolution than CT. As might result in this difference. However, the prevalence in our study was less than Dörffel et al. (29) who used ultrasonography and had a follow-up to identify that 14.9% of patients with AP demonstrated splenic vein thrombosis, in which the thrombosis was demonstrated at 3 days to 7 weeks (average, 10–14 days) after the diagnosis of AP. Our prevalence was also less than Mortelé et al. (23) who found that the prevalence of splenic vein thrombosis was 19%. In their study, 54 patients experienced previous episodes of AP, the time between the diagnosis of AP and CT evaluation in some patients was longer (up to 21 days), and some of the patients had a second or third follow-up. The reasons for the lower prevalence in our study may be that all of the patients were the first onset and underwent MRI scan within one week after the onset and there was no follow-up. Thus, the time between the MRI/CT evaluation and the diagnosis of AP, the number of pancreatitis episodes, and the follow-up are all important to evaluate the splenic vein in AP. Xiao et al. (22) had once described the invasion of vascular wall in AP on MRI, but they had not consecutive study. We also found the splenic phlebitis/venous wall invasion and arteritis/arterial wall invasion on MRI. Our study suggested that MRI may be a good method to assess the vessels in AP.
In our study, the prevalence of splenic vascular involvement was positively correlated with the severity of AP based on both the MRSI and the New Revised Classification of AP 2012, which better predict the local and systemic conditions of patients and the prognoses. Our result suggested that the splenic vascular involvement in AP might help to assess the severity of AP in the clinical and imaging setting. Mortelé et al. (23) found that the presence of splenic vein thrombosis was significantly correlated with the severity of pancreatitis. Furthermore, we found that the splenic vascular involvement was much more common in necrotizing AP than in edematous AP, which was in conformity with the reports (28-30).
Splenic morphological abnormalities in AP are rarely and typically reported as reviews or case reports (7,8,31). We only found splenomegaly and splenic infarction in this study. Splenomegaly could present in pancreatic disease uncomplicated by cirrhosis or other disorders (32). Tsushima et al. (6) had found that there was transient splenomegaly in AP. In our study, 16.7% of AP patients presented the finding of splenomegaly, whose prevalence was increased with the severity of AP increasing and was also increased in necrotizing AP than in edematous AP, but they did not reach statistical significance (P>0.05). Mostly, the etiology of splenomegaly is probably portal hypertension (25). In pancreatic disorders, the compression of portal/splenic vein or the vein thrombosis might cause splenic venous obstruction and cause the pressure in spleen to increase, eventually leading to the congestive splenomegaly (6,32-34). No matter in mild, moderate, or severe AP and in edematous or necrotizing AP, the pancreas might swell and compress the splenic vein, possibly related to those results.
Conventional sequences provide a limited evaluation of tissue characteristics, however, IVIM MR imaging plays a more and more important role in evaluating microstructural characteristics noninvasively. It has been widely used in many organs, such as the brain, breast, hepatic, pancreatic, gastric and rectal lesions (14-16,35-37). AP could cause secondary micro-changes in several organs (38), it had been found that there were some pathological changes in splenic tissue under the microscopy in severe AP (5).
In this study, we found that the splenic f value was higher in AP than that in the control group and that it was higher in AP patients with splenomegaly than in those without splenomegaly (P<0.05). The f value represents the fraction of the signal originating from perfusion and is expected to reflect the fractional blood volume of capillaries (14-16). Our study demonstrated that the splenic perfusion fraction degree increased in AP and that it increased more in those AP patients with splenomegaly. To our best knowledge, this is the first study which evaluates the splenic changes in AP by using IVIM, there is no data of those parameters. Tutcua et al. (38) has found that AP causes significant perfusion changes in the hepatic tissue and a significant increase in blood volume in the lobes of liver and that the perfusion changes might depend on the local inflammation process and the effect of systemic mediators. As their study mentioned, in AP, the local inflammation might also spread to the spleen through the splenorenal ligament and finally along the splenic red pulp, the local inflammation and the effect of systemic mediators in AP might be also related to the perfusion changes in the splenic tissue. Additionally, our result showed that the splenic perfusion fraction degree increased more in those AP patients with splenomegaly. It might suggest that the effect of local inflammation and the effect of systemic mediators in the splenic tissue are more significant in those AP patients with splenomegaly.
One limitation of this study was that the splenic involvement might not be caused by pancreatitis, we eliminated the potential confounders when we selected the cases by excluding the patients with diseases that could cause the spleen and splenic vascular involvement. Another limitation was that the diagnosis of splenomegaly used a practical and simplified way rather than calculating the splenic volume, we used the well-correlated parameter with splenic volume to minimize the bias.
In conclusion, splenic vascular involvement and splenomegaly are common in AP. Splenic vascular involvement helps the evaluation of the severity of AP in the clinical and imaging setting. Quantitative analysis of the spleen with IVIM shows that AP may cause perfusion changes in splenic tissue, especially in those with splenomegaly.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board and Ethics Committee of Affiliated Hospital of North Sichuan Medical College approved this study [No. 2017ER(A)002] and waived patients’ informed consent because it was a retrospective study.
References
- Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol 2002;34:167-76. [Crossref] [PubMed]
- Rau BM. Outcome determinants in acute pancreatitis. Am J Surg 2007;194:S39-44. [Crossref]
- Xiping Z, Yan P, Xinmei H, Guanghua F, Meili M, Jie N, Fangjie Z. Effects of dexamethasone and Salvia miltiorrhizae on the small intestine and immune organs of rats with severe acute pancreatitis. Inflammation 2010;33:259-66. [Crossref] [PubMed]
- Shi C, Zhao X, Lagergren A, Sigvardsson M, Wang X, Andersson R. Immune status and inflammatory response differ locally and systemically in severe acute pancreatitis. Scand J Gastroenterol 2006;41:472-80. [Crossref] [PubMed]
- Xiping Z, Ruiping Z, Binyan Y, Li Z, Hanqing C, Wei Z, Rongchao Y, Jing Y, Wenqin Y, Jinjin B. Protecting effects of a large dose of dexamethasone on spleen injury of rats with severe acute pancreatitis. J Gastroenterol Hepatol 2010;25:302-8. [Crossref] [PubMed]
- Tsushima Y, Tamura T, Tomioka K, Okada C, Kusano S, Endo K. Transient splenomegaly in acute pancreatitis. Br J Radiol 1999;72:637-43. [Crossref] [PubMed]
- Lankisch PG. The spleen in inflammatory pancreatic disease. Gastroenterology 1990;98:509-16. [Crossref] [PubMed]
- Fishman EK, Soyer P, Bliss DF, Bluemke DA, Devine N. Splenic involvement in pancreatitis: spectrum of CT findings. AJR Am J Roentgenol 1995;164:631-5. [Crossref] [PubMed]
- Amano Y, Oishi T, Takahashi M, Kumazaki T. Nonenhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging 2001;26:59-63. [Crossref] [PubMed]
- Stimac D, Miletić D, Radić M, Krznarić I, Mazur-Grbac M, Perković D, Milić S, Golubović V. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007;102:997-1004. [Crossref] [PubMed]
- Kim YK, Ko SW, Kim CS, Hwang SB. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging 2006;24:1342-9. [Crossref] [PubMed]
- Ji YF, Zhang XM, Mitchell DG, Li XH, Chen TW, Li Y, Bao ZG, Tang W, Xiao B, Huang XH, Yang L. Gastrointestinal tract involvement in acute pancreatitis: initial findings and follow-up by magnetic resonance imaging. Quant Imaging Med Surg 2017;7:641-53. [Crossref] [PubMed]
- Chi XX, Chen TW, Huang XH, Yang L, Tang W, Wáng YX, Xiao B, Zhang XM. Magnetic resonance imaging of retroperitoneal interfascial plane involvement in acute pancreatitis. Quant Imaging Med Surg 2016;6:250-8. [Crossref] [PubMed]
- Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology 2008;249:748-52. [Crossref] [PubMed]
- Wáng YXJ, Deng M, Li YT, Huang H, Leung JCS, Chen W, Lu PX. A Combined Use of Intravoxel Incoherent Motion MRI Parameters Can Differentiate Early-Stage Hepatitis-b Fibrotic Livers from Healthy Livers. SLAS Technol 2017. Epub ahead of print. [Crossref] [PubMed]
- Wurnig MC, Donati OF, Ulbrich E, Filli L, Kenkel D, Thoeny HC, Boss A. Systematic analysis of the intravoxel incoherent motion threshold separating perfusion and diffusion effects: Proposal of a standardized algorithm. Magn Reson Med 2015;74:1414-22. [Crossref] [PubMed]
- Li XH, Zhu J, Zhang XM, Ji YF, Chen TW, Huang XH, Yang L, Zeng NL. Abdominal MRI at 3.0 T: LAVA-Flex compared with conventional fat suppression T1-weighted images. J Magn Reson Imaging 2014;40:58-66. [Crossref] [PubMed]
- Ly JN, Miller FH. MR imaging of the pancreas: a practical approach. Radiol Clin North Am 2002;40:1289-306. [Crossref] [PubMed]
- Pamuklar E, Semelka RC. MR imaging of the pancreas. Magn Reson Imaging Clin N Am 2005;13:313-30. [Crossref] [PubMed]
- Tang MY, Chen TW, Huang XH, Li XH, Wang SY, Liu N, Zhang XM. Acute pancreatitis with gradient echo T2*-weighted magnetic resonance imaging. Quant Imaging Med Surg 2016;6:157-67. [Crossref] [PubMed]
- Sarr MG, Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Tsiotos GG, Vege SS. The new revised classification of acute pancreatitis 2012. Surg Clin North Am 2013;93:549-62. [Crossref] [PubMed]
- Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH. Magnetic resonance imaging for local complications of acute pancreatitis: a pictorial review. World J Gastroenterol 2010;16:2735-42. [Crossref] [PubMed]
- Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol 2004;52:67-72. [Crossref] [PubMed]
- Chi XX, Zhang XM, Chen TW, Huang XH, Yang L, Tang W, Xiao B. The normal transverse mesocolon and involvement of the mesocolon in acute pancreatitis: an MRI study. PLoS One 2014;9:e93687. [Crossref] [PubMed]
- Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol 2001;11:80-95. [Crossref] [PubMed]
- Bezerra AS, D'Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol 2005;184:1510-3. [Crossref] [PubMed]
- Rezai P, Tochetto SM, Galizia MS, Yaghmai V. Splenic volume model constructed from standardized one-dimensional MDCT measurements. AJR Am J Roentgenol 2011;196:367-72. [Crossref] [PubMed]
- Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas 2013;42:1251-4. [Crossref] [PubMed]
- Dörffel T, Wruck T, Rückert RI, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas 2000;21:126-33. [Crossref] [PubMed]
- Easler J, Muddana V, Furlan A, Dasyam A, Vipperla K, Slivka A, Whitcomb DC, Papachristou GI, Yadav D. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol 2014;12:854-62. [Crossref] [PubMed]
- Toussi HR, Cross KS, Sheehan SJ, Bouchier-Hayes D, Leahy AL. Spontaneous splenic rupture: a rare complication of acute pancreatitis. Br J Surg 1996;83:632. [Crossref] [PubMed]
- Gregory PB, Klatskin G. Splenomegaly in uncomplicated biliary tract and pancreatic disease. Gastroenterology 1972;62:436-40. [PubMed]
- Madsen MS, Petersen TH, Sommer H. Segmental portal hypertension. Ann Surg 1986;204:72-7. [Crossref] [PubMed]
- Takase M, Suda K, Suzuki F, Nakamura T, Futagawa S. A histopathologic study of localized portal hypertension as a consequence of chronic pancreatitis. Arch Pathol Lab Med 1997;121:612-4. [PubMed]
- Ji C, Chen L, Guan W, Guo T, Zhang Q, Liu S, He J, Zhou Z. Intravoxel incoherent motion magnetic resonance imaging in assessing histopathological features of gastric cancers: initial findings. Transl Cancer Res 2017;6:1129-40. [Crossref]
- Li YT, Cercueil JP, Yuan J, Chen W, Loffroy R, Wáng YX. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg 2017;7:59-78. [Crossref] [PubMed]
- Yan C, Pan X, Chen G, Ge W, Liu S, Li M, Nie L, He J, Zhou Z. A pilot study on correlations between preoperative intravoxel incoherent motion MR imaging and postoperative histopathological features of rectal cancers. Transl Cancer Res 2017;6:1050-60. [Crossref]
- Tutcu S, Serter S, Kaya Y, Kara E, Neşe N, Pekindil G, Coşkun T. Hepatic perfusion changes in an experimental model of acute pancreatitis: evaluation by perfusion CT. Eur J Radiol 2010;75:203-6. [Crossref] [PubMed]


