Posterior reversible encephalopathy syndrome after intrathecal methotrexate infusion: a case report and literature update
Introduction
The first definition of posterior reversible encephalopathy syndrome (PRES), dates back to 1996, when Hinchey described a reversible clinical-radiological syndrome characterized by acute onset of headache, nausea, dizziness, changes in consciousness, convulsions and transient visual disturbances such as cortical blindness and white matter oedema mainly localised in the occipital-parietal lobes (1,2). Magnetic resonance imaging (MRI) shows bilateral grey and white matter abnormalities predominantly in the posterior regions of the cerebral hemispheres and cerebellum (3). PRES is the result of multitude of factors and has been associated with numerous medical conditions. Hypertension and immunosuppressive or cytotoxic drugs have been reported to be the most significant causes of PRES (4).
Methotrexate (MTX) is a commonly used chemotherapeutic agent in children with cancer. It can cause leukoencephalopathy, which ranges from acute and reversible to recurrent, chronic or irreversible and rarely fatal (5). The pathophysiology of leukoencephalopathy is not well understood. Acute encephalopathy generally develops within 5–14 days after intrathecal (IT) or high dose (HD) MTX and resolves within a week without permanent neurological sequelae (3).
The occurrence of PRES in the paediatric population has been recently recognized, however, because of the sparse paediatric cases ever reported, both paediatricians and radiologists may be less familiar with this entity. Herein, we report a case of a 13-year old boy with Burkitt lymphoma/leukaemia, who presented with posterior leukoencephalopathy 24 hours after IT MTX infusion; a thorough literature review is obtained.
Case presentation
A 13-year-old boy was referred to our department with a probable diagnosis of intestinal B-cell lymphoma. Bone marrow aspiration and immunophenotype revealed bone marrow infiltration of more than 70% blasts, which were positive for CD45, CD19, CD20 and HLA-DR, DP, DQ. Cytogenetics showed the presence of t(8;14) (q24;q32) translocation, that confirmed the diagnosis of Burkitt lymphoma/leukaemia and the boy was treated according to the UKCCSG NHL 903 chemotherapy protocol (six cycles of prednisolone, vincristine, adriamycin, cytarabine, MTX along with IT infusions of hydrocortisone, MTX and cytarabine). Two months after the end of the treatment he presented with local relapse, without any evidence of bone marrow disease. Therefore, he started treatment according to the BFM NHL 2004 chemotherapeutic regimen (dexamethasone, ifosfamide, cyclophosphamide, high-dose MTX, cytarabine, teniposide, adriamycin along with IT infusions of hydrocortisone, MTX and cytarabine).
Three months later, while he was still continuing with his treatment, and 24 hours after IT MTX infusion, he presented with an episode of generalized tonic-clonic seizures and developed severe headache. Neurological examination revealed mydriasis, while physically he remained stable and generally in good condition. Cerebrospinal fluid (CSF) had been tested routinely for central nervous system (CNS) disease on the day prior to this episode while he was under IT MTX. The results from the CSF specimen were normal. Blood pressure was slightly elevated (129/82 mmHg) (95th percentile both for systolic and diastolic according to his height) and the heart rate was about 90 beats/min. Biochemical and electrolyte screening were within normal limits. He was treated with intravenous (IV) bolus administration of 5 mg diazepam after 5 min. Due to the continuation of seizures for over 20 minutes IV phenytoin was initiated with 20 mg/kg of body weight with success. However three hours later two new episodes of generalized tonic-clonic seizures occurred. His blood pressure was elevated further (170/120 mmHg), while his neurological status gradually deteriorated and the child became lethargic. He was treated with the additional administration of IV bolus midazolam 0.2 mg/kg, dexamethasone and furosemide for the reduction of cerebral oedema.
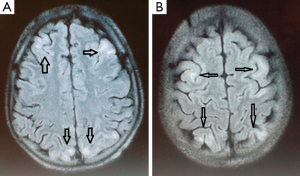
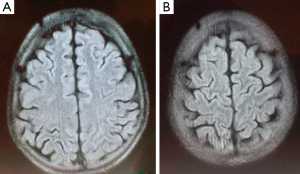
Over the ensuing two days his neurological examination steadily improved and his mental status restored to normal. Brain MRI, performed on the sixth day after the cessation of the convulsions, demonstrated frontal and parietal-occipital cortical and subcortical hyperintense lesions on fluid-attenuated inversion-recovery (FLAIR) and T2W images with no contrast enhancement on T1W images. On apparent diffusion coefficient (ADC) maps the lesions showed increased ADC values, representing vasogenic oedema (Figure 1). Two weeks later, a follow-up MRI scan was performed showing no abnormality in those areas (Figure 2). On examination, no neurological deficit was detected. Despite intensive chemotherapy the patient died due to the progression of the underlying disease and the severe long term bone marrow suppression.
Discussion
PRES is a clinical-radiological entity characterised clinically by seizures, severe headache, mental status instability and visual disturbances such as cortical blindness and hallucinations. Somnolence and lethargy are the key clinical features on presentation. Visual abnormalities may present as the disease evolves. Hypertension is typically presented from the outset, though normal blood pressure has been reported in some cases. The term “posterior reversible leukoencephalopathy” may not be accurate since the outcome is not always reversible, the lesions are not always located to the posterior regions of the brain and it may affect not only the white but also the grey matter (6). In the recent years, the broad use of MRI has led to an increasing number of identified cases of PRES. However, the incidence of PRES in children remains still low (7,8). It seems that there is a significant female predominance, which may reflect some of the immunological causes of this entity (9). A thorough literature search found that up to now few cases of PRES in children with haematological malignancies have been reported (Table 1) (10-17).

Full table
The pathophysiology of PRES remains not only unclear but also enigmatic. One reason for this is its multifactorial nature. PRES reflects a cerebral vasogenic oedema and the main pathophysiology is that of neurotoxicity. It is speculated that PRES is due to a breakdown in the blood brain barrier leading to a leakage of fluid to the intermediate space of the cerebral parenchyma which in turn generates vasogenic oedema (18). Immunosuppressant drugs have also a cytotoxic effect on the vascular endothelium. Whether vasospasm per se contributes to ischemia it remains unclear. Alterations in systemic blood pressure may affect the vessels of the brain, however sympathetic auto-regulatory mechanisms can control and reverse this effect (18,19).
PRES can occur in the absence of hypertension in 20–40% of the cases (20). Additionally, patients with severe hypertension are reported to have a milder vasogenic oedema than normotensive patients (20). This result would have been unexpected, if the mechanism of PRES was exclusively severe hypertension with dysfunctional auto-regulation mechanisms (21). Since several patients with PRES have normal blood pressure, endothelial damage and default of the blood-brain barrier could explain the pathogenesis of the syndrome (22). However, it is still unclear whether this phenomenon is the direct effect of an autoimmune process or an indirect complication of the applied treatment for the underlying disease.
Immunosuppressive and cytotoxic drugs have been accused to cause either direct endothe-lial damage or disruption of the endothelial cell integrity. The latter leads to release of endo¬thelin, a protein that causes vasoconstriction of the cerebral vessels. This as a domino phenomenon triggers mild and reversible ischemia and oedema of the white matter (23). Moreover, chemotherapeutic agents (MTX, L-asparaginase, adriamycin, cyclophosphamide, cytosine arabinoside, vincristine) contribute to PRES also by inducing or exacerbating hypertension due to corticosteroids treatment or renal dysfunction (24). Erythropoietin and certain colony stimulating factors such as G-CSF have been associated with PRES as well. This syndrome has also been reported to be associated with newer targeted therapies such as antivascular endothelial growth factor (VEGF) agents (bevacizumab), anti-CD20 antibodies (rituximab), tyrosine kinase inhibiting (TKI) agents (sorafenib, sunitinib, pazopanib) and, most recently, with anti-cytotoxic T-cell lymphocyte antigen 4 (CTLA-4) agents (ipilimumab) (24).
Throughout the treatment of acute childhood leukemia, PRES may occur as a neurological complication. Induction chemotherapy regimens of acute lymphoblastic leukaemia (consisting of systemic steroids, repetitive intrathecal MTX, vincristine, and L-asparaginase) may comprise predisposing factors of PRES. Asparaginase-induced toxicity or MTX-induced encephalopathy should be differentiated from PRES. High-dose MTX of 1.5–8 g/m2 and age above 10 years old are associated with an increased risk for acute encephalopathy in children with ALL (5). In our case, the child presented with recurrent generalized tonic-clonic seizures followed by lethargy and hypertension, in accordance with the symptoms described by other authors (12,13). MTX-induced encephalopathy shows a tendency to involve more often the cerebral white matter and also has a tendency to occur in a certain time period, usually few weeks after intrathecal MTX infusion. These characteristics may prove helpful in the differential diagnosis between chemotherapy-induced leukoencephalopathy and PRES (25).
Risk factors for PRES in paediatric cancer patients are hypertension (not necessarily acute) and remission induction chemotherapy. Common complications of treatment in malignancies, such as hypertension, systemic inflammatory response, sepsis, hyperviscosity syndrome, coagulopathy and electrolyte imbalance, could affect negatively the prognosis and alter the course of the disease (21). Most of the patients develop PRES during the induction phase of treatment (26). The reason for this timing is not clarified but it does not seem to be random. Possibly unidentified systemic factors associated with the induction phase of treatment predispose patients to PRES perhaps through an autoimmune response. A recent study showed that serum lactate dehydrogenase (LDH), a marker of endothelial dysfunction, had a statistically significant elevation at the onset of PRES toxicity in cancer patients receiving chemotherapy (27). Immunological processing seems to be further involved in the genesis of PRES. Cytokine production in the early phase of Guillain-Barré Syndrome could play a role in altering capillary permeability and cause breakdown of the blood brain barrier (28).
The typical features of PRES are not present in all paediatric PRES patients. Kwon et al. reported 12 patients that presented with seizures (42%), visual disturbances (33%), headache (17%), or altered mental status (8%) (29). İncecik et al. detected that the most common clinical features were: altered mental status, seizures and headache (30). Other symptoms were nausea, vomiting and blurred vision (30). The findings from the physical examination, in the majority of cases, are non-specific for the disease. With the exception of brisk deep tendon reflexes, particularly of the lower extremities, and possibly extensor plantar responses, the rest of the clinical examination is relatively unimpressive (18).
Typically, PRES presents as widespread, usually reversible vasogenic oedema, predominantly in the sub-cortical white matter of the occipital and parietal lobes (Figure 1). In large retrospective studies, this is the most common pattern of oedema (31). The predilection for the posterior cerebral areas is thought to be due to the fact that the circulation there is more susceptible to impaired auto-regulation and to the genesis of vasogenic oedema in the setting of hypertension (32). With the advent of neuroimaging, PRES has become more easily identifiable.
The findings from the brain MRI are typical for this disease and include hyperintense signal in T2 weighted and FLAIR images at the cortical-subcortical level. FLAIR and DWI images can be helpful to distinguish vasogenic from cytotoxic oedema, the second being rather unusual in PRES lesions (12). Vasogenic oedema typically shows isointensity or hypointensity in DWI and hyperintensity on the ADC map, while cytotoxic edema shows hyperintensity in DWI and hypointensity on the ADC map (21). The presence of vasogenic oedema within the posterior arterial watershed region suggests a reversible process with a favourable prognosis. In contrast, cytotoxic oedema beyond the posterior circulation is atypical findings for PRES with a less favourable outcome (29).
Although rarely, atypical imaging findings of unusual distribution patterns, presence of cytotoxic oedema, infarction, hemorrhage, and contrast enhancement have been recently reported (33,34).
Moreover, the median time of clinical resolution in a recent study of children with PRES was 4.8 days (range, 1.5–14 days) (35), consistent with the result of adults (5.3 days) (8). MRI remains the examination of choice for establishing the diagnosis of PRES. Various conditions must be differentiated from PRES, such as infectious encephalitis, acute disseminated encephalomyelitis, progressive multifocal leukoencephalopathy (PML), vasculitis, cerebral venous sinus thrombosis, and ischemic stroke (32).
The treatment approach is to restore the underlying cause of PRES. Early diagnosis and immediate correction of the cause of PRES are the keys to successful management. Symptom-directed management consisting of antiepileptic drugs, removal of potentially triggering factors, and of course, treatment of the underlying cause that leads to hypertension (35).
No consensus has yet been achieved about which patients should receive antiepileptic drugs for seizures after PRES (25,36). Anticonvulsant therapy is recommended even after a single seizure (9). Morris et al. recommended continuation of the antiepileptic treatment for 3–6 months in uncomplicated cases (14). They also recommended an anticonvulsant regimen for at least 12 months in patients with recurrent seizures and abnormal findings on electroencephalogram (14). Lucchini et al. advised anticonvulsant therapy for 12 months in patients with brain damage (12).
Withdrawal of the potential triggering agents may not always be accompanied by a favourable outcome. Some authors have treated PRES by initiating cyclophosphamide and few have continued ongoing treatment with cyclophosphamide and reported no recurrence (37). Conversely, other authors have reported a case of worsening of symptoms when a patient with PRES received cyclophosphamide (38). High-dose corticosteroids can cause hypertension. However, removal of steroids could also be proven harmful unless the blood pressure is not controlled (22).
The prognosis of PRES is often benign provided that early diagnosis is made and management is accurate and in time. In these cases restoration is usually seen several days or weeks after the onset of symptoms (8). However, delayed diagnosis and improper management may result in permanent brain insult, even death (23,35).
The presence of cytotoxic oedema is the strongest unfavourable prognostic factor for the disorder as it can lead to irreversible cerebral insult (39). A significant number of patients develop epilepsy despite of clinical and radiological evidence of recovery (14). A minimum follow up period of two years with clinical surveillance, EEG and MRI is needed in order to evaluate furthermore the prognosis of PRES (12).
Conclusions
Early recognition of PRES as a complication during various diseases and therapies in childhood may facilitate precise diagnosis and appropriate treatment in a timely manner, conferring a good prognosis. Given the widespread use of steroids and other medications used in oncology, it is important to recognize PRES as a rare but usually reversible complication, especially in paediatric patients receiving treatment for acute lymphoblastic leukaemia. Appropriate treatment should be initiated accordingly after ruling out other causes, which could result in a similar clinical presentation like cortical venous thrombosis, CNS hemorrhage, CNS leukemic infiltration, and encephalitis. We should emphasize the importance of close monitoring of blood pressure in children with critical neurological illness and predisposing factors for PRES. High suspicion should be raised in these patients when hypertension is detected, especially in the context of acute consciousness alteration or convulsions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500. [Crossref] [PubMed]
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 2000;21:1199-206. [PubMed]
- Endo A, Fuchigami T, Hasegawa M, Hashimoto K, Fujita Y, Inamo Y, Mughisima H. Posterior reversible encephalopathy syndrome in childhood: report of four cases and review of the literature. Pediatr Emerg Care 2012;28:153-7. [Crossref] [PubMed]
- Utsumi K, Amemiya S, Lizuka M, Lino Y, Katayama Y. Acute posterior leukoencephalopathy in a patient with nephrotic syndrome. Clin Exp Nephrol 2003;7:63-6. [Crossref] [PubMed]
- Dufourg MN, Landman-Parker J, Auclerc MF, Schmitt C, Perel Y, Michel G, Levy P, Couillault G, Gandemer V, Tabone MD, Demeocq F, Vannier JP, Leblanc T, Leverger G, Baruchel A. Age and high-dose methotrexate are associated to clinical acute encephalopathy in FRALLE 93 trial for acute lymphoblastic leukemia in children. Leukemia 2007;21:238-47. [Crossref] [PubMed]
- Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J 2005;35:83-90. [Crossref] [PubMed]
- Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol 2007;28:1320-7. [Crossref] [PubMed]
- Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008;65:205-10. [PubMed]
- Arzanian MT, Shamsian BSh, Karimzadeh P, Kajiyazdi M, Malek F, Hammoud M. Posterior Reversible Encephalopathy Syndrome in Pediatric Hematologic-Oncologic Disease: Literature Review and Case Presentation. Iran J Child Neurol 2014;8:1-10. [PubMed]
- Ulu EM, Toere HG, Bayrak A, Gungor D, Coskun M. MRI of central nervous system abnormalities in childhood leukaemia. Diagn Interv Radiol 2009;15:86-92. [PubMed]
- Hourani R, Abboud M, Hourani M, Khalifeh H, Muwakkit S. L-asparaginase-induced posterior reversible encephalopathy syndrome during acute lymphoblastic leukemia treatment in children. Neuropediatrics 2008;39:46-50. [Crossref] [PubMed]
- Lucchini G, Grioni D, Colombini A, Contri M, De Grandi C, Rovelli A, Conter V, Masera G, Jankovic M. Encephalopathy Syndrome in Children With Hemato-Oncological Disorders Is Not Always Posterior and Reversible. Pediatr Blood Cancer 2008;51:629-33. [Crossref] [PubMed]
- Inaba H, Khan RB, Laningham FH, Crews KR, Pui CH, Daw NC. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol 2008;19:178-84. [Crossref] [PubMed]
- Morris EB, Laningham FH, Sandlund JT, Khan RB. Posterior Reversible Encephalopathy Syndrome in Children With Cancer. Pediatr Blood Cancer 2007;48:152-9. [Crossref] [PubMed]
- Umeda K, Yoshida M, Suzuki N, Endo M, Sato A, Hori H, Isogai M, Matsumoto K, Hara JI, Hasegawa D, Hashii Y, Chayama K, Miyaji R, Nishimura S, Tanizawa A, Uami I, Horibe K, Wakazono Y, Yagi K. Complications in the central nervous system during chemotherapy for childhood acute lymphoblastic leukemia: JACLS ALL-02 study. Rinsho Ketsueki 2007;48:204-11. [PubMed]
- Cooney MJ, Bradley WG. MD, Symko SC, Patel ST, Groncy PK. Hypertensive Encephalopathy: Complication in Children Treated for Myeloproliferative Disorders—Report of Three Cases. Radiology 2000;214:711-16. [Crossref] [PubMed]
- Bernini JC, Fort DW, Griener JC, Kane BJ, Chappell WB, Kamen BA. Aminophylline for methotrexate-induced neurotoxicity. Lancet 1995;345:544-7. [Crossref] [PubMed]
- Appachu MS, Purohit S, Lakshmaiah KC, Kumari BS, Appaji L. Posterior reversible encephalopathy syndrome in pediatric acute leukemia: Case series and literature review. Indian J Med Paediatr Oncol 2014;35:79-82. [Crossref] [PubMed]
- Rajasekhar A, George TJ. Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: a case report and review of the literature. Oncologist 2007;12:1332-35. [Crossref] [PubMed]
- Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447-55. [Crossref] [PubMed]
- Yoon SD, Cho BM, Oh SM, Park SH, Jang IB, Lee JY. Clinical and Radiological Spectrum of Posterior Reversible Encephalopathy Syndrome. J Cerebrovasc Endovasc Neurosurg 2013;15:206-13. [Crossref] [PubMed]
- Gatla N, Annapureddly N, Sequeira W, Jolly M. Posterior Reversible Encephalopathy Syndrome in Systemic Lupus Erythematosus. J Clin Rheumatol 2013;19:334-40. [Crossref] [PubMed]
- Grossbach AJ, Abel TJ, Hodis B, Wassef SN, Greenlee JD. Hypertensive posterior reversible encephalopathy syndrome causing posterior fossa edema and hydrocephalus. J Clin Neurosci 2014;21:207-11. [Crossref] [PubMed]
- Khurana A, Dasanu C. Posterior reversible encephalopathy syndrome due to targeted agens: vemurafinib among suspects! J Oncol Pharm Pract 2015;21:443-50. [Crossref] [PubMed]
- Kim SJ, Im SA, Lee JW, Chung NG, Cho B, Kim HK. Predisposing Factors of Posterior Reversible Encephalopathy Syndrome in Acute Childhood Leukemia. Pediatr Neurol 2012;47:436-42. [Crossref] [PubMed]
- Nakajima N, Ueda M, Nagayama H, Yamazaki M, Katayama Y. Posterior Reversible Encephalopathy Syndrome due to Hypercalcemia Associated with Parathyroid Hormone-related Peptide: A Case Report and Review of the Literature. Intern Med 2013;52:2465-68. [Crossref] [PubMed]
- Fitzgerald RT, Wright SM, Samant RS, Kumar M, Ramakrishnaiah RH, Van Hemert R, Brown AT, Angtuaco EJ. Elevation of serum lactate dehydrogenase at posterior reversible encephalopathy syndrome onset in chemotherapy-treated cancer patients. J Clin Neurosci 2014;21:1575-8. [Crossref] [PubMed]
- Rigamonti A, Basso F, Scaccabarozzi C, Lauria G. Posterior reversible encephalopathy syndrome as the initial manifestation of Guillain-Barre syndrome: case report and review of the literature. J Peripher Nerv Syst 2012;17:356-60. [Crossref] [PubMed]
- Kwon S, Koo J, Lee S. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol 2001;24:361-64. [Crossref] [PubMed]
- İncecik F, Hergüner MÖ, Yıldızdaş D, Yılmaz M, Mert G, Horoz ÖO, Altunbaşak Ş. Posterior reversible encephalopathy syndrome due to pulse methylprednisolone therapy in a child. Turk J Pediatr 2013;55:455-7. [PubMed]
- Ni J, Zhou Li-Xin, Hao H-L, Liu Q, Yao M, Li M, Peng B, Cui LY. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging 2011;21:219-24. [Crossref] [PubMed]
- Stevens CJ, Heran MKS. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol 2012;85:1566-75. [Crossref] [PubMed]
- Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome:Imaging and clinical features. Am J Neuroradiol 2009;30:1371-79. [Crossref] [PubMed]
- Sharma A, Whitesell RT, Moran KJ. Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology 2010;52:855-63. [Crossref] [PubMed]
- Chen TH, Lin WC, Tseng YH, Tseng CM, Chang TT, Lin TJ. Posterior Reversible Encephalopathy Syndrome in Children: Case Series and Systematic Review. J Child Neurol 2013;28:1378-86. [Crossref] [PubMed]
- Siebert E, Spors B, Bohner G, Endres M, Liman TG. Posterior reversible encephalopathy syndrome in children: radiological and clinical findings - a retrospective analysis of a German tertiary care center. Eur J Paediatr Neurol 2013;17:169-75. [Crossref] [PubMed]
- Bag AK, Cure JK, Sullivan JC, Roberson GH. Central variant of posterior reversible encephalopathy syndrome in systemic lupus erythematosus: new associations? Lupus 2010;19:225-6. [Crossref] [PubMed]
- Varaprasad IR, Agrawal S, Prabu VN, Rajasekhar L, Kanikannan MA, Narsimulu G. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus. J Rheumatol 2011;38:1607-11. [Crossref] [PubMed]
- Zhang Y, Zhou J, Chen Y. Posterior reversible encephalopathy syndrome in a child with cortico-resistant nephritic syndrome: a case report and review of literature. Int J Clin Exp Pathol 2014;7:4433-7. [PubMed]


