In vivo quantification of bone mineral density of lumbar vertebrae using fast kVp switching dual-energy CT: correlation with quantitative computed tomography
Introduction
Osteoporosis is a common disease that causes low bone mass and bone microstructural destruction. Patients with osteoporosis have increased bone fragility and a high risk of fracture, which results in substantial family and socioeconomic burdens (1,2). Bone mineral density (BMD) is the main measurement of the amount of bone and is directly related to osteoporosis (3-5). Both dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) are regarded as reference standards for the measurement of BMD. DXA is used to measure the areal BMD (aBMD) in units of g/cm2. However, the early changes in bone mass in osteoporosis first occur in trabecular bone, due to the inner trabecular bone being more active metabolically and thus more sensitive to changes in BMD (6).
QCT has some advantages over DXA because of its three-dimensional nature and the opportunistic exploitation of routine CT scans. In recent years, QCT has increasingly been used instead of DXA to assess BMD of the lumbar spine (7,8). Uniquely, QCT can provide separate BMD values for trabecular and cortical bone and measure true volumetric BMD (vBMD) in units of mg/cm3 (9,10), which not only reflects changes of bone mineral content in the vertebrae more accurately than DXA, but also predicts incident vertebral fractures more accurately than DXA (11-13). Phantomless QCT is gaining in popularity due to the convenience of requiring no calibration phantoms and the benefit of allowing for opportunistic BMD measurements. Currently, there are three main techniques of phantomless QCT. The first technique uses values of internal tissues (muscle and fat) as references for calibration when calculating BMD; the second allows for measurement of BMD without a phantom being scanned with each patient, as long as the CT modality is calibrated periodically; and the third estimates BMD by performing material decomposition (MD) using dual-energy CT (DECT) (14).
The recently introduced DECT method provides not only monochromatic images, but also accurate MD images by gemstone spectral imaging (GSI) (15,16). In vivo BMD can be estimated by quantifying the base materials using MD algorithms. Like QCT, DECT can also support separate measurements of the BMD of cortical and trabecular bone (5). Notably, the radiation exposure dose of DECT with the volume-based adaptive statistical iterative reconstruction (ASiR-V) technique is equal to or lower than that of conventional CT (17-19). DECT also has other potential advantages, including detection of bone marrow edema or tumor infiltration. Compared with the first two techniques of phantomless QCT using conventional CT, DECT with the ASiR-V technique has no barriers to its use as a routine scan for lumbar examination, which suggests a promising future for BMD quantitation based on DECT.
Previous studies on the measurement of BMD by DECT are controversial and inadequate. van Hamersvelt et al. found a strong correlation between BMD measured by DECT and DXA (20), but their study was based on phantoms rather than patients. Conversely, other studies indicated a lack of correlation between DECT-derived and DXA-derived BMD, although these studies included fewer than 50 participants (21,22). Mei et al. reported that compared with QCT, DECT-based hydroxyapatite (HAP)-specific BMD quantitation had a high level of accuracy in vitro (23); however, its performance in vivo has yet to be evaluated. Recently, Roski et al. demonstrated the feasibility of phantomless in vivo dual-layer DECT-based HAP-specific BMD assessment, although only 33 patients were included and only HAP-specific BMD was measured (24). To date, few studies have investigated the use of the new-generation fast kVp switching DECT.
Some studies have demonstrated that absorption by any type of tissue can be determined by the proportions of its base materials, and with appropriate selection of the base materials their densities can reflect the content of the actual material in the tissue (19,25,26). The main components of the vertebral body include bone minerals (HAP/calcium), water, red marrow, yellow marrow (mainly fat), and collagen. Therefore, we hypothesized that compared with phantom-calibrated QCT, 256-row fast kVp switching DECT was feasible and accurate for phantomless BMD quantification in vivo. In our study, the density of four base material pairs were evaluated: calcium (water) [DCa(Wa)]; HAP (water) [DHAP(Wa)]; calcium (fat) [DCa(Fat)]; and HAP (fat) [DHAP(Fat)].
Methods
Study design and selection of subjects
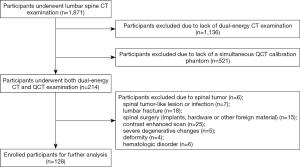
Our study was approved by the institutional ethics committee of the First Affiliated Hospital of Hunan University of Chinese Medicine (no. HN-LL-KY-2019003016). Owing to the retrospective nature of this study, the requirement for informed consent was waived. Data from subjects who underwent DECT lumbar examinations between July 2018 and February 2019 were collected. The subjects’ demographic characteristics [age, sex, and body mass index (BMI)] before scanning were recorded. The exclusion criteria were no simultaneous QCT calibration phantom; spinal tumor; spinal tumor-like lesions or infection; lumbar fracture; spinal surgery (implants, hardware, or other foreign material); contrast-enhanced scan; severe degenerative changes; deformity; and hematologic disorder (12,14). Finally, 128 consecutive participants were enrolled. Figure 1 is a flowchart of the study following the guidelines of Standards for Reporting of Diagnostic Accuracy. The sample size consideration is shown in Supplementary Material A1.
DECT and phantom-calibrated QCT scan protocols
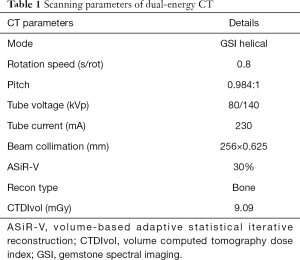
In our study, all lumbar examinations of the enrolled participants were performed simultaneously with a bone density calibration (BDC) phantom placed beneath the spine, to avoid additional radiation exposure. CT imaging of the spine was performed with a Revolution GSI CT scanner (GE Medical Systems, Milwaukee, WI, USA). Table 1 shows the detailed scanning parameters of DECT, and Supplementary Material A2 shows the radiation exposure dose consideration of DECT using the ASiR-V technique (27).
Full table
The BDC phantom (QRM, Moehrendorf, Germany) contained 6 cylindrical inserts, each with a diameter of 1.8 cm and an exact value of 0, 98.8, 201.9, 390.5, 599.1, and 793.7 mg/cm3 HAP, respectively. All phantom-calibrated QCT analyses were performed according to the ISCD consensus, and BMD was measured at L1 (lumbar level 1) and L2 (lumbar level 2) (13). The DECT scanner undergoes a ‘fast calibration’ protocol every week to ensure correct CT values of water and no artifacts. The accuracy and precision of the QCT devices based on DECT are tested every week using the same scan protocol (for details see Supplementary Material A3).
Post-processing of DECT and phantom-calibrated QCT image data
The MD data and QCT data were evaluated in random order by Y.H. (8 years of experience in musculoskeletal radiology) and H.G. (10 years of experience), respectively, who were independent and blinded to each other’s results. The raw image data were transmitted to an advanced workstation (ADW4.6; GE Medical Systems). As they had lower image noise and a higher contrast-to-noise ratio (CNR) than 120-kVp CT images, but equal CT values, 70-keV monochromatic images were used to measure the CT values of both the vertebrae and the phantom in this study (28,29). The reproducibility and accuracy of spinal QCT using different scan protocols is shown in Supplementary Material A3 and Table S1.
For further analysis, 5-mm axial images were viewed. In the central level of the lumbar vertebral body, the region of interest (ROI) was drawn along the inner edge of the vertebral body to calculate the average CT attenuation value (HU), avoiding the cortical bone and the vertebral venous plexuses posteriorly (Figure 2). DCa(Wa), DHAP(Wa), DCa(Fat), and DHAP(Fat) were also separately measured in the MD images. The main component of bone is HAP [chemical formula Ca10(PO4)6(OH)2]. The relative atomic weight of calcium (Ca) is 40. Because there are 10 Ca atoms in HAP, the amount of Ca is 400, and the relative molecular weight of HAP is 1,004. When the Ca content of HAP is known, the HAP content can be estimated according to Eq. [1]. A circular ROI with a fixed diameter of 10 mm was marked in the center of each insert in the BDC phantom, and the mean CT number of each insert was measured (Figure 2). A linear regression Eq. [2] could be calculated with the known HAP content and CT attenuation value of each insert in the phantom, and then the CT attenuation value of the vertebral body could be converted into BMD through Eq. [2] (23).

[1]
[2]
ρCa-H20 and ρCa-Fat are the density of calcium (water) and calcium (fat), respectively.
β and c are the coefficient and intercept of the linear formula, respectively.
Statistical analysis
SPSS statistical analysis software (v.20.0; IBM) and R software (version 3.5.1; http://www.Rproject.org) were used for statistical analysis. Continuous variables were compared using the Wilcoxon test or Student’s t test. The data were checked for homogeneity and normality using the Levene’s and Shapiro-Wilk tests, respectively.
Linear regression analysis was used to assess the relationship between DECT- and QCT-derived BMD. Linear regression models were quantitatively evaluated using adjusted R-square, normalized mean squared error (NMSE), and relative error (RE) (30,31). NMSE was calculated by 10-fold cross-validation, according to Eq. [3]:
[3]
yi is the true value of BMD in test set, y is the mean of true value of BMD in test set.
RE was calculated in two ways according to Eq. [4]:
[4]
RE is generally given as a percentage, ∆-Absolute error (predicted value minus true value), and L-True value (QCT-derived BMD). For RElinear, the adjusted predicted BMD from the linear regression model was used to calculate ∆. The adjusted predicted value for a case is the predicted value when that case is excluded from the calculation of the regression coefficients (corresponding to leave-one-out cross-validation). For REdirect, the DHAP (DCa converted into DHAP) measured in MD images was considered as the predicted value to calculate ∆.
Bland-Altman analysis was conducted to assess agreement between the adjusted predicted BMD (derived from linear regression calibration) and QCT-derived BMD (32).
Statistical significance was indicated by two-sided P<0.05.
Results
Characteristics of the subjects
The demographic characteristics of the subjects are summarized in Table 2. The data were analyzed at both the participant level (n=128, the average of L1–L2 per participant was used for analysis) and the vertebral level (n=128, L1 and L2, separately).

Full table
Relationship between QCT-derived and DECT-derived BMD
The results of this section are summarized in Figures 3 and 4, and Table S2.
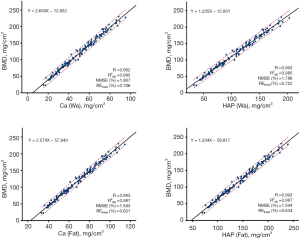
The results for the participant level are as follows. The REdirect of DCa(Wa), DHAP(Wa), DCa(Fat), and DHAP(Fat) was −56.7%, −63.2%, −37.5%, and −47.8%, respectively. Figure 3 shows the significant positive correlations between the densities of the four base materials [DCa(Wa), DHAP(Wa), DCa(Fat) and DHAP(Fat)] and QCT-derived BMD (R=0.992–0.993, adjusted R2 =0.985–0.987, all P<0.001). All the linear models showed good predictive capability of BMD (NMSE =1.6–1.8%, RElinear = 0.6–0.7%).
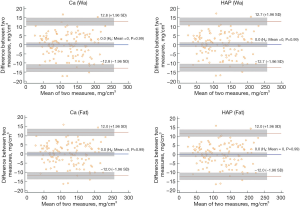
Additionally, the agreements between BMD measurements based on linear regression calibrated DECT and QCT were assessed with Bland-Altman plots (Figure 4). For all four base materials, the mean differences were zero (all P>0.05); most of the differences lay between ±1.96 SD, and there were no clear trends, which indicated high agreement between both measurements.
The results for the vertebral level were as good as those based on the participant level. The densities of the four base materials [DCa(Wa), DHAP(Wa), DCa(Fat), and DHAP(Fat)] and QCT-derived BMD were highly correlated (R = 0.991–0.993, adjusted R2 = 0.983–0.987, all P<0.001). All of the linear models showed good predictive capability of BMD (NMSE = 1.7–2.1%, RElinear = 0.7–0.9%).
All of the above results indicated the robustness and accuracy of our models. Furthermore, DCa(Fat) and DHAP(Fat) were found to demonstrate relatively similar and optimal predictive capability for QCT-derived BMD (both: adjusted R2 =0.987, NMSE =1.6%, RElinear =0.6%).
Discussion
Osteoporosis is a chronic condition that imposes significant health, social, and economic burdens worldwide because of its prevalence. However, with early medical intervention, the condition can be treated. Consequently, early diagnosis of osteoporosis has attracted considerable attentions (1). Our study demonstrated that fast kVp switching DECT enabled accurate BMD quantification of the lumbar spine in vivo without the need for phantom-calibration, and the DCa(Fat)-specific and DHAP(Fat)-specific densities showed relatively similar and optimal predictive capability.
Controversy surrounds the associations between DECT-derived and DXA-derived BMD found in previous studies. Van Hamersvelt et al. inferred that DECT allowed accurate BMD quantification in vitro by using two validated anthropomorphic phantoms with material-specific known concentrations (20); Similarly, Wait et al. indicated that DECT was more sensitive than DXA in detecting changes of BMD, and that the BMD values measured by DECT and DXA were highly correlated (5). On the other hand, DECT-derived and DXA-derived BMD values have been reported to have a low correlation. In the study by Wesarg et al., 29 cadaver specimens were evaluated, and the authors observed only a moderate linear correlation of the BMD measurements between DECT and DXA (33). Moreover, Wichmann et al. analyzed 160 lumbar vertebrae in 40 participants and reported a lack of correlation between the BMD results derived from DECT and DXA (21). In our study, with QCT serving as the reference standard, strong linear correlations were observed between the densities of the four base materials [DCa(Wa), DHAP(Wa), DCa(Fat) and DHAP(Fat)] and phantom-calibrated BMD.
There are several possible reasons for the discrepancies among studies. Firstly, DXA measures aBMD in g/cm2, but QCT measures vBMD in mg/cm3, which allows for differentiation of trabecular and cortical bone. Secondly, the results of the studies conducted on phantoms were better than those of the studies conducted on participants, which might relate to fewer interfering factors in the phantom studies, such as individual patient differences. Thirdly, our research was conducted on 128 participants of different sexes, ages, and BMI from a clinic, but only 40 participants were included in the study by Wichmann et al., which makes our results more reliable. Fourthly, we used DECT with the new generation of spectral imaging technology in MD; this gave wider detector coverage, better image quality, and lower exposure dosage, all of which are helpful for accurate quantification of BMD. In general, the results of our study may be more accurate and convincing.
Recently, Mei et al. demonstrated that with a radiation exposure of ≥50 mA, a high correlation was found between BMD values measured with DECT and QCT (23,24). They investigated BMD quantitation both in phantoms with known HAP concentrations and in participants, showing the measurements to be highly accurate. Taken together, the findings suggest that phantomless BMD quantitation based on DECT imaging is feasible and could be applied clinically. It must be emphasized that our study was conducted on the lumbar vertebrae of 128 participants, and the densities of four base material pairs [DCa(Wa), DHAP(Wa), DCa(Fat) and DHAP(Fat)] were analyzed, with significant correlations found between BMD values measured by DECT and QCT. Our results suggest that material-specific measurements are an adequate alternative for the detection of patients with low BMD in routine clinical practice.
Few studies have investigated the relationship between BMD predictive capability and different MD technology. Several methods (adjusted R2, RE, and NMSE) were applied in the linear model evaluation in this study, and good linearity, stability and consistency were shown at both the participant level and the vertebral level. Furthermore, DCa(Fat) and DHAP(Fat) had relatively similar and optimal predictive capability. Materials display energy-dependent X-ray absorption at different kilovoltage peak levels. With DECT, materials can be further differentiated through the differences in attenuation by applying different X-ray spectra (34-36). Our results indicated that the stability of the MD technique for the four-base material pairs may differ. However, the stability was relatively similar and optimal between Ca(Fat) and HAP(Fat), which brought the most accurate BMD predictive results calibrated by regression equation.
To date, there have been two methods of predicting BMD by DECT: direct quantification based on measurements in MD images, and indirect quantification through linear regression calibration. However, few studies have compared the accuracy of these methods. It is interesting that the direct quantification method was not as accurate as we expected. The REs of the four-base material pairs in the direct method were large and varied, which would result in biased estimates and makes the method difficult to apply clinically. In contrast, the REs of the four-base material pairs with the indirect method were small and similar, and the agreement between the indirect method and QCT was good, which implied great clinical value. These results demonstrated that the DECT MD should not be considered as a measurement of the true content of a certain material, but only reflects the relative content and the change trend of the base materials. The estimated values of the direct method needed to be calibrated by linear regression equation for clinical use.
Study limitations
This study has some limitations that need to be addressed. Firstly, there are various components of the vertebral body [red marrow, yellow marrow (mainly fat), water, collagen, and bone minerals] and the present noninvasive BMD/fat quantification methods, including DECT, DXA, conventional QCT, and MRI, can only provide rough estimates. However, chemically analyzed density measurement is not applicable in vivo. Secondly, only the lumbar spine was analyzed in this study, and the values for the thoracic vertebrae, proximal femur, and distal radius, which are also at risk of fragility fractures, should be considered. Thus, in future study, we will aim to address predictive capability of the BMD in those sites.
In conclusion, 256-row dual-energy CT with fast kVp switching technique enabled accurate in vivo BMD quantification of lumbar spine without phantom-calibration. DCa(Fat) and DHAP(Fat) had relatively similar and optimal predictive capability, which may open up the possibilities of using this DECT technique for osteoporosis assessment in clinical practice.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (grant no. 81603482), Hunan Natural Science Foundation (grant no. 2016JJ6115), China Postdoctoral Science Foundation (grant no. 2017M622586), Key Discipline Construction Project of Hunan University of Chinese Medicine (grant no. 4901-020000200806).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-367). The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval for this study was given by the institutional ethics committee of the First Affiliated Hospital of Hunan University of Chinese Medicine (no. HN-LL-KY-2019003016), and the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown C. Osteoporosis: Staying strong. Nature 2017;550:S15-7. [Crossref] [PubMed]
- Sànchez-Riera L, Carnahan E, Vos T, Veerman L, Norman R, Lim SS, Hoy D, Smith E, Wilson N, Nolla JM, Chen JS, Macara M, Kamalaraj N, Li Y, Kok C, Santos-Hernandez C, March L. The global burden attributable to low bone mineral density. Ann Rheum Dis 2014;73:1635-45. [Crossref] [PubMed]
- Berry SD, Samelson EJ, Pencina MJ, McLean RR, Cupples LA, Broe KE, Kiel DP. Repeat bone mineral density screening and prediction of hip and major osteoporotic fracture. Jama 2013;310:1256-62. [Crossref] [PubMed]
- Wu Y, Guo Z, Fu X, Wu J, Gao J, Zeng Q, Fu H, Cheng X. The study protocol for the China Health Big Data (China Biobank) project. Quant Imaging Med Surg 2019;9:1095-102. [Crossref] [PubMed]
- Wait JM, Cody D, Jones AK, Rong J, Baladandayuthapani V, Kappadath SC. Performance Evaluation of Material Decomposition With Rapid-Kilovoltage-Switching Dual-Energy CT and Implications for Assessing Bone Mineral Density. AJR Am J Roentgenol 2015;204:1234-41. [Crossref] [PubMed]
- Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom 2008;11:123-62. [Crossref] [PubMed]
- Link TM, Lang TF. Axial QCT: clinical applications and new developments. J Clin Densitom 2014;17:438-48. [Crossref] [PubMed]
- Gerety EL, Hopper MA, Bearcroft PW. The reliability of measuring the density of the L1 vertebral body on CT imaging as a predictor of bone mineral density. Clin Radiol 2017;72:177.e9-15. [Crossref] [PubMed]
- Liu Y, Carrino JA, Dash AS, Chukir T, Do H, Bockman RS, Hughes AP, Press JM, Stein EM. Lower Spine Volumetric Bone Density in Patients With a History of Epidural Steroid Injections. J Clin Endocrinol Metab 2018;103:3405-10. [Crossref] [PubMed]
- Kwon D, Kim J, Lee H, Kim B, Han H, Oh H, Kim M, Yoon H, Lee B, Eom K. Quantitative computed tomographic evaluation of bone mineral density in beagle dogs: comparison with dual-energy x-ray absorptiometry as a gold standard. J Vet Med Sci 2018;80:620-8. [Crossref] [PubMed]
- Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res 2008;23:1326-33. [Crossref] [PubMed]
- Löffler MT, Jacob A, Valentinitsch A, Rienmüller A, Zimmer C, Ryang YM, Baum T, Kirschke JS. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol 2019;29:4980-9. [Crossref] [PubMed]
- Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, Rosen HN, Weber DR, Zemel BS, Shepherd JA. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. Journal of Clinical Densitometry 2019;22:453-71. [Crossref] [PubMed]
- American College of Radiology (2018) ACR–SPR–SSR practice parameter for the performance of musculoskeletal quantitative computed tomography (QCT). Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf, Accessed 7 Nov 2018.
- Pan J, Yan L, Gao H, He Y, Zhong Z, Li P, Zhang Y, Guo Y, Liao L, Zhou S, Zhang K. Fast kilovoltage (KV)-switching dual-energy computed tomography hydroxyapatite (HAP)-water decomposition technique for identifying bone marrow edema in vertebral compression fractures. Quant Imaging Med Surg 2020;10:604-11. [Crossref] [PubMed]
- Mallinson PI, Coupal TM, McLaughlin PD, Nicolaou S, Munk PL, Ouellette HA. Dual-Energy CT for the Musculoskeletal System. Radiology 2016;281:690-707. [Crossref] [PubMed]
- Li W, Li A, Wang B, Niu X, Cao X, Wang X, Shi H. Automatic spectral imaging protocol and iterative reconstruction for radiation dose reduction in typical hepatic hemangioma computed tomography with reduced iodine load: a preliminary study. Br J Radiol 2018;91:20170978. [Crossref] [PubMed]
- Lv P, Zhou Z, Liu J, Chai Y, Zhao H, Guo H, Marin D, Gao J. Can virtual monochromatic images from dual-energy CT replace low-kVp images for abdominal contrast-enhanced CT in small- and medium-sized patients? Eur Radiol 2019;29:2878-89. [Crossref] [PubMed]
- Zheng S, Dong Y, Miao Y, Liu A, Zhang X, Wang B, Ge Y, Liu Y, Wang S. Differentiation of osteolytic metastases and Schmorl's nodes in cancer patients using dual-energy CT: advantage of spectral CT imaging. Eur J Radiol 2014;83:1216-21. [Crossref] [PubMed]
- van Hamersvelt RW, Schilham AMR, Engelke K, den Harder AM, de Keizer B, Verhaar HJ, Leiner T, de Jong PA, Willemink MJ. Accuracy of bone mineral density quantification using dual-layer spectral detector CT: a phantom study. Eur Radiol 2017;27:4351-9. [Crossref] [PubMed]
- Wichmann JL, Booz C, Wesarg S, Kafchitsas K, Bauer RW, Kerl JM, Lehnert T, Vogl TJ, Khan MF. Dual-energy CT-based phantomless in vivo three-dimensional bone mineral density assessment of the lumbar spine. Radiology 2014;271:778-84. [Crossref] [PubMed]
- Booz C, Hofmann PC, Sedlmair M, Flohr TG, Schmidt B, D'Angelo T, Martin SS, Lenga L, Leithner D, Vogl TJ, Wichmann JL. Evaluation of bone mineral density of the lumbar spine using a novel phantomless dual-energy CT post-processing algorithm in comparison with dual-energy X-ray absorptiometry. Eur Radiol Exp 2017;1:11. [Crossref] [PubMed]
- Mei K, Schwaiger BJ, Kopp FK, Ehn S, Gersing AS, Kirschke JS, Muenzel D, Fingerle AA, Rummeny EJ, Pfeiffer F, Baum T, Noel PB. Bone mineral density measurements in vertebral specimens and phantoms using dual-layer spectral computed tomography. Sci Rep 2017;7:17519. [Crossref] [PubMed]
- Roski F, Hammel J, Mei K, Baum T, Kirschke JS, Laugerette A, Kopp FK, Bodden J, Pfeiffer D, Pfeiffer F, Rummeny EJ, Noël PB, Gersing AS, Schwaiger BJ. Bone mineral density measurements derived from dual-layer spectral CT enable opportunistic screening for osteoporosis. Eur Radiol 2019;29:6355-63. [Crossref] [PubMed]
- Dong Y, Zheng S, Machida H, Wang B, Liu A, Liu Y, Zhang X. Differential diagnosis of osteoblastic metastases from bone islands in patients with lung cancer by single-source dual-energy CT: advantages of spectral CT imaging. Eur J Radiol 2015;84:901-7. [Crossref] [PubMed]
- Fischer MA, Gnannt R, Raptis D, Reiner CS, Clavien PA, Schmidt B, Leschka S, Alkadhi H, Goetti R. Quantification of liver fat in the presence of iron and iodine: an ex-vivo dual-energy CT study. Invest Radiol 2011;46:351-8. [Crossref] [PubMed]
- Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology 2010;254:145-53. [Crossref] [PubMed]
- Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S. Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 2011;259:257-62. [Crossref] [PubMed]
- Yamada Y, Jinzaki M, Hosokawa T, Tanami Y, Abe T, Kuribayashi S. Abdominal CT: an intra-individual comparison between virtual monochromatic spectral and polychromatic 120-kVp images obtained during the same examination. Eur J Radiol 2014;83:1715-22. [Crossref] [PubMed]
- Engelke K, Glüer CC. Quality and performance measures in bone densitometry: part 1: errors and diagnosis. Osteoporos Int 2006;17:1283-92. [Crossref] [PubMed]
- Chen KY. Combining linear and nonlinear model in forecasting tourism demand. Expert Systems with Applications 2011;38:10368-76. [Crossref]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Wesarg S, Kirschner M, Becker M, Erdt M, Kafchitsas K, Khan MF. Dual-energy CT-based assessment of the trabecular bone in vertebrae. Methods Inf Med 2012;51:398-405. [Crossref] [PubMed]
- Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, Fink C, Weckbach S, Lenhard M, Schmidt B, Flohr T, Reiser MF, Becker CR. Material differentiation by dual energy CT: initial experience. Eur Radiol 2007;17:1510-7. [Crossref] [PubMed]
- Sauter AP, Hammel J, Ehn S, Achterhold K, Kopp FK, Kimm MA, Mei K, Laugerette A, Pfeiffer F, Rummeny EJ, Pfeiffer D, Noel PB. Perfusion-ventilation CT via three-material differentiation in dual-layer CT: a feasibility study. Sci Rep 2019;9:5837. [Crossref] [PubMed]
- Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol 2009;193:47-54. [Crossref] [PubMed]